Past Issues
Synthesis and Characterization of a Silver Nanoparticle- Based Material
Antoine Demange, Solenne Fleutot*
IJL – UMR 7198 – CNRS-University of Lorraine, Nancy, France
*Corresponding author: Solenne Fleutot, IJL – UMR 7198 – CNRS-University of Lorraine, Nancy, France, E-mail: [email protected] Received: December 14, 2019 Published: August 26, 2020
ABSTRACT
In this work, we synthesize and characterize a material made from silver nanoparticles stabilized thanks to a biocompatible polymer. The first step of the study concerns the synthesis of silver nanoparticles colloidal suspension stabilized by polyvinylpyrrolidone (PVP) and his characterization by different techniques (TEM, DLS) leading to the production of nanoparticles of different sizes and shapes. Following this characterization, the second step is the development of a structured material in fibers and/or balls by electrospinning from the Ag@PVP solution. This material was characterized by SEM and EDS demonstrating the formation of Ag@PVP fibers and / or beads.
KEYWORDS: Silver Nanoparticles, Colloidal Suspension, PVP, Electrospinning
INTRODUCTION
The objective of this work is the development of a biocompatible hybrid material consisting of a polymer (poly (vinylpyrrolidone) labeled PVP) and silver nanoparticles. A biocompatible material is a material that does not interfere with and does not degrade its biological environment. In particular, it does not release a toxic molecule for living tissue. These materials are then called biomaterials. Materials formed from PVP and containing silver particles will be shaped by electrospinning, a method which allows the development of fibers and/or particles.
The electrospinning is a method of producing fibers that uses electrical energy to pull polymer or molten polymer wires up to fibers of few hundred nanometers diameter. This process suitable for the production of materials using complex molecules or polymers doesn't require the use of coagulation or very high temperatures to produce fibers, particles or films from the solution. Finally, this method guarantees the absence of traces of solvent in the final product with its evaporation on the way between the needle and the collector.
MATERIALS AND METHODS
Materials
Absolute ethanol (CAS: 64-17-5), silver nitrate (CAS: 7761-88-8), poly(vinylpyrrolidone) PVP (CAS: 9003-39-8, Mw ~ 360,000).
Syntheses
There are many ways to synthesize silver nanoparticles. However, in all of the syntheses whose precursor is a silver salt, a reducing agent will be necessary. It can be chemical in nature and in this case the standard reaction potential must be greater than 0 V. The couple (Ag+ → Ag0) having an E0 = +0.799V, it is therefore possible to use a large number of chemical reducers such as for example sodium borohydride (E0 = -0.481V), sodium citrate (E0 = -0.180V), hydrazine (E0 = -0.230V) and many more. Here we are choosing ethanol to reduce silver ions.
The reaction involved is [1]:
2Ag+ + C2H5O → 2Ag0 + CH3CHO
In 40mL of absolute ethanol,1g of silver nitrate then 1.0g of PVP are dissolved to room temperature [2]. Once the silver nitrate and PVP are dissolved, the solution is heated at 60°C, preventing the evaporation of ethanol. When the solution changes color and becomes yellow- orange, heating is stopped and the solution is stirred on a cold plate for 1 hour. The ethanal redox/ethanol couple having a standard potential (E0 = -0.190V) lower than that of the Ag/Ag + couple (E0 = 0.799V), it will play the role of reducing agent to synthesize the silver nanoparticles. Polyvinylpyrrolidone acts as a stabilizer to allow the silver nanoparticles to remain in the Ag form. Once the nanoparticles have been reduced, the non-binding doublets of the ketone functions and of the nitrogen contained in the PVP will create interactions stabilizing Ag [3]. Silver nanoparticles surrounded by PVP (shell) and noted Ag@PVP will then be created.
Electrospinning
The solution is introduced into a syringe which is placed in a constant rate syringe pump. The syringe is fitted with a polished hypodermic needle to obtain a flat end. The collector on which the final product will land is grounded while the positive terminal is installed on the syringe needle. Constant voltage is applied.
The parameters influencing the synthesized fibers are the polymer concentration, the applied voltage, the flow of the syringe, the diameter of the needle, the distance between the needle and the collector and finally the volatility of the solvent. Time has no influence on the organization of the material but it only defines the quantity and the thickness of material which will be deposited on the collector.
For practical reasons and in order to avoid a large number of variables, the flow rate is fixed at 0.794 mL/h-1, the needle is a 23 gauge (0.574 mm) and the needle-collector distance is 15 cm for the entire study. Finally, the solvent used in this study will always be absolute ethanol.
The only variables will therefore be the polymer concentration and the applied voltage. The percentage of PVP in ethanol will be 5%, 2.5% and 1.25% by mass respectively and the applied voltages will be 5kV, 10kV, 15kV and 20kV.
The samples will be noted according to the example below:
X N1 N2
with : X the PVP content N1 the Silver content
content [4] N2 the applied voltage for electrospinning
And: PVP with A corresponds to 5% PVP in ethanol, B 2.5% and C 1.25%
Silver content with 5 represents the mass ratio 1/5 Ag / PVP, 10 corresponds to a ratio of 1/10 and 20 corresponds to a ratio of 1/20.
For example, the sample noted A10 5 corresponds to 5% PVP in ethanol, a ratio 1/10 Ag/PVP and an applied voltage of 5 kV for electrospinning.
Characterization Methods
Silver nanoparticles will be characterized for their optical properties using a UV-Visible spectrometer. Transmission Electron Microscopy TEM (CM200 - FEI operating at 200 kV with a point resolution of 0.27 nm) will characterize the shape and size of silver particles. The hydrodynamic radius of the silver nanoparticles will be obtained using Dynamic Light Diffraction DLS (ZETASIZER Nano ZS from Malvern instrumental).
The material obtained after electrospinning will be characterized by a Scanning Electron Microscope SEM (Quanta 650 FEG of FEI) to obtain the topology and finally by Energy Dispersive Analysis (EDS) to confirm the presence of silver in the PVP.
RESULTS AND DISCUSSION
The silver Ag is gray but normally, at the nanoscale, for the effects of surface plasmons, plasmonicsilver nanoparticles have a different color depending on their shape, size and the possible aggregates formation. Plasmons are the oscillations of free electrons within the metal which are the consequence of the formation of a dipole due to electromagnetic waves. The light waves oscillate and carry with it the free electrons at the same frequency. This coupling only occurs if the frequency of light is less than or equal to that of plasma and is the highest among the plasma resonance frequencies.
UV-Visible spectroscopy analysis allows determining approximately the size of the silver nanoparticles. The size obtained by this technique can be compared with that measured using TEM or DLS.
A solution color evolution is observed over time (Figure 1). This evaluates from a pale yellow to a dark orange color. Indeed, the silver nanoparticles at room temperature and at light will tend to grow and/or form aggregates. Even if the nanoparticles are very stable when they are within a PVP matrix, it is necessary to keep them away from light and at low temperature to limit their evolution over time.
Figure 1: B10 at 60°C after 4 min, 35 min, 47 min, 109 min.
UV-Visible
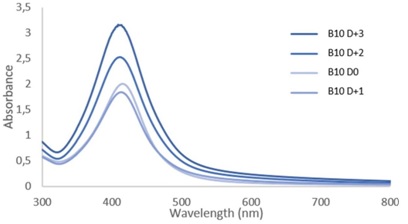
The analyzed samples here are from the same synthesis. Once the synthesis was finished, a part of the solution was placed directly in the fridge (notation D0). Others parts were left in the light and at room temperature for few days (1, 2 or 3 days) to observe the evolution of these samples over time (notation D+1, D+2 and D+3).
UV-Visible spectra show us several aspects. We can observe an increase of the absorbance indicating that the concentration of silver nanoparticles increased in 3 days between D0 and D+3 (Figure 2). The concentration increase shows that at the end of the heat period in synthesis (D0) all the silver ions haven't reduced to metallic silver.
Figure 2: Absorbance spectra of sample B10 at D0 and after 1, 2 or 3 days (D+1, D+2 and D+3 respectively).
The theoretical absorbance curves of model nanoparticles with a well-defined size reported in the literature [5] also allow the particle size to be evaluated using λmax. For our sample, the size of the nanoparticles can be approximated around 30 nm. However, compared to these same theoretical curves, the obtained results show a significant half-height width, describing a large particle size distribution. Finally, we can observe that the curves fall back to zero absorbance for a long wavelength (around 750-800nm). According to the literature [6], this could be explained by the growth of faceted, hexagonal or even triangular nanoparticles in small proportion or even the formation of aggregates.
Figure 2 shows that the B10 sample has a higher concentration of silver nanoparticles at D0 by compared to D+1 unlike the overall evolution. A possible explanation lies in keeping samples in the fridge. The D0 sample was taken out and used for analyzes more often than the D+1. This sample D0 could therefore have evolved differently depending on the frequent temperature fluctuations to which it was subjected.
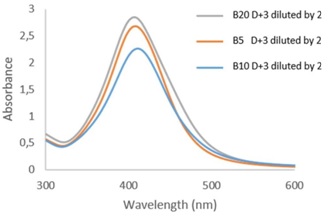
Regarding the influence of the Ag/PVP mass ratio (Figure 3), a higher absorbance is obtained for the sample containing only 1/20 of Ag by comparison with that containing 1/10 or 1/5. Several hypotheses could explain this phenomenon. The first is that the 1/20 synthesis stayed a little longer on the hot plate. Maintaining at 60°C could have favored the germination of small nanoparticles and thus greatly increase the absorbance at λmax. The second hypothesis is that from a 1/10 ratio the aggregates are formed more easily and therefore strongly decrease the absorbance at λmax (corresponding to the small sizes of nanoparticles) and increase that around 450-550 nm (corresponding to larger nanoparticles). This hypothesis would be confirmed using of TEM analyzes.
Figure 3: Absorbance spectra of samples B5,B10 and B20 after 3 days.
TEM
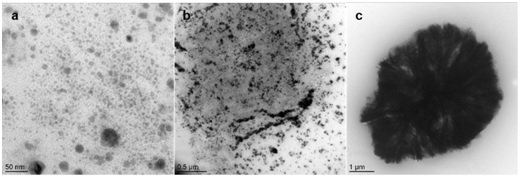
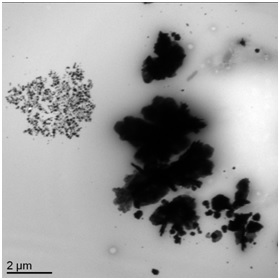
TEM micrographs allow to observe a large dispersion for sample B10 ranging from a few nanometers to a few micrometers. The silver nanoparticles form large aggregates confirming the information given by UV-Visible. We observe for this sample (Figure 4) areas where the silver nanoparticles are small and dispersed (a), other areas where the particles are very concentrated, less dispersed and begin to form aggregates (b) and finally, large aggregates of several micrometers in diameter (c).
Figure 4: TEM micrographs of sample B10.
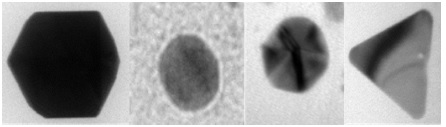
The results also show the presence of several types of nanoparticles: faceted, spherical, triangular and hexagonal particles (Figure 5). The implemented synthesis protocol doesn't lead to a single morphology of the nanoparticles. The morphology is influenced by the growth of nanoparticles. The spherical and faceted nanoparticles are observed from 10 to 50 nm and the hexagonal and triangular nanoparticles for sizes on the order of 50 nm to several µm.
Figure 5: Different nanoparticle morphologies.
Significant differences in particle size and shape can be observed within a sample (Figure 6) with well-separated populations of small particles and large aggregates. These observations are in agreement with the UV-Visible results showing the presence of small particles by the λmax and large aggregates in the area ranging from about 450 to 550 nm for the same sample as that shown in Figure 6 (sample B10 D+3).
Figure 6: TEM micrograph of sample B10 D+3.
According to the literature [6], a colloidal solution of spherical silver nanoparticles will be yellow. In the case of this study, the dark orange color of the solution (Figure 1) could be explained by the diversity of shapes and sizes highlighted thanks to the TEM and UV-Visible analyzes.
DLS
The DLS gives indications on the size of the particles in solution. The DLS determines the hydrodynamic radius of the particle (accounting for both the radius of the particle + the interaction of the surface with the solvent). Here the DLS will allow a measurement of silver nanoparticles, from the PVP shell into surface and interactions with the solvent. So we would expect to have much larger particle sizes compared to those obtained by UV-Visible or TEM for the same sample. However, it depends on the behavior in solution of Ag@PVP systems.
Thus, if the TEM micrograph in Figure 6 shows that the particles range from a few nanometers to several micrometers, the DLS makes it possible to discern several particle populations and their relative proportions in the sample for a given analysis. The sample collection analyzed by DLS as the analysis duration can influence the obtained results. It is therefore necessary to repeat this analysis several times for better accuracy of results.
For a non-homogeneous Ag@PVP colloidal solution, the sampling of the analyzed solution can favor one population over another. Sedimentation can also take place during the analysis, leading to an evolution of the results over time. The presence of large PVP chains can also hinder the proper functioning of DLS and limit the collection of all data.
Characterization of the Electrospun Material SEM
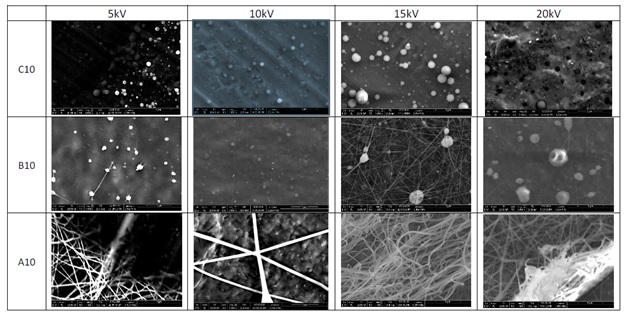
SEM pictures taken after electrospinning of Ag@PVP solutions (Figure 7) at different concentrations of PVP show a great influence on the shape of the material obtained on the collector. At low concentrations, PVP will lead to the formation of small particles. The higher the concentration, the more the material will tend to form fibers to the detriment of the particles. For the B10 series samples, we can see that the balls are deformed (hollow or bumps). This can be linked to the solvent which does not evaporate before collection and is trapped within the beads. It will therefore have to pierce and deform the particles to be able to escape.
Figure 7: SEM images showing the evolution of the shaping of the material by electrospinning as a function of the applied voltage and the PVP content.
The change in the voltage applied during electrospinning influences the size of the particles (Figure 7). 500 particles were measured on SEM images using software to determine an average size. The results obtained for the C10 series are reported in Table 1. The higher the applied voltage, the greater the particles size increases. In addition, the standard deviation tends to increase with the applied voltage because the higher the tension, the more there will be a risk of instability during electrospinning. These instabilities will tend to result in a greater diversity of beads sizes.
|
|
C10 5 |
C10 10 |
C10 15 |
C10 20 |
|
Meandiameter (µm) |
0.680 ± 0.130 |
0.745 ± 0.109 |
1.110 ± 0.488 |
1.565 ± 0.387 |
EDS
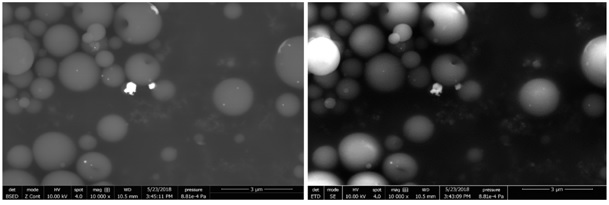
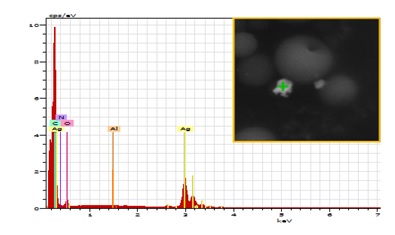
EDS analyzes confirm the presence of silver particles on the surface or within the polymer beads. This surface analysis allows to characterize only a reduced depth of sample. The distribution of silver particles on the surface or within the polymer beads is therefore not discernible by this method. According to the data, we can observe with the BSED detector (Figure 8 right) the presence of the heaviest elements such as silver highlighted. The image obtained in secondary electron with SEM (Figure 8 left) gives an account of the topography of the sample.
Figure 8: SEM images with ETD (left) and BSED (right) detectors.
Figure 9: EDS spectrum with analysis object in insert.
CONCLUSION
The synthesis of silver nanoparticles was carried out with various sizes and shapes. The process of production by electrospinning has led to the formation of materials composed of beads, fibers or a mixture by simple variation of the concentration of polymer in solution. The colloidal silver solution could also be sprayed in the form of balls in preformed fibers or meshes of Ag@PVP allowing to add new properties to a already existing material.
Conflicts of interest: The author declares no conflict of interest.
REFERENCES
- Mtimet I. (2011). Elaboration de surfaces biocides contenant des nanoparticules d’argent, Ph.D. thesis.
- Kendouli S, khalfallah O, Sobti N, Bensouissi A, Avci A, et al. (2014). Modification of cellulose acetate nanofibers with PVP/Ag addition. Materials Science in Semiconductor Processing. (28):13-19.
- Huang HH, Ni XP, Loy GL, Chew CH, Tan KL, et al. (1996). Photochemical Formation of Silver Nanoparticles in Poly(N-vinylpyrrolidone). Langmuir. 4(12):909-912.
- Baptista AC, Botas AM, Almeida APC, Nicolau AT, Falcão BP, et al. (2015). Down conversion photoluminescence on PVP/Ag-nanoparticles electrospun composite fibers. Optical Materials. (39):278-281.
- Data NanoComposix : UV-Vis report, dilution corrected Spectrum of sample Silver Nano, Agilent 8453 UV-Visible spectrometer, www.nanocomposix.com.
- Pacioni NL, Borsarelli CD, Rey V, Veglia AV. (2015). Synthetic Routes for the Preparation of Silver Nanoparticles. Chapter in Silver Nanoparticle Application.
Copyright: Fleutot S, et al. ©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Fleutot S (2020). Synthesis and Characterization of a Silver Nanoparticle-Based Material. Nanoparticle 2(1): 05.
 Abstract
Abstract  PDF
PDF