Past Issues
Nanoparticles Toxicity and Biocompatibility Tests
Solenne Fleutot
IJL – UMR 7198 – CNRS-University of Lorraine, Nancy, France
*Corresponding author: Solenne Fleutot, IJL – UMR 7198 – CNRS-University of Lorraine, Nancy, France, E-mail: [email protected] Received: September 11, 2020 Published: September 28, 2020
ABSTRACT
The toxicity of inorganic nanoparticles is a key issue for their development in the biomedical field. There are many tests to determine if a compound is harmful to the body, but some are applied more than others to nanoparticles. This is the case of the MTT and XTT tests which count living cells or of the TUNEL test which analyzes DNA fragments and detects cellular apoptosis. Cytometry techniques – flow and laser scanning- allow examining the evolution of the cell- life cycle while the damage suffered by DNA will rather be analyzed by the comets method.
KEYWORDS: Toxicity tests; Nanoparticles; SPIONs
INTRODUCTION
Nanomaterials and more particularly nanoparticles have experienced increasing interest in recent decades thanks to the development of new methods of synthesis, characterization, and analysis. Due to the nanometric scale, nanomaterials will have a very high surface/volume ratio which allows differentiation of the physicochemical properties (magnetic, optical, catalytic properties, etc.) compared to those of the bulk material.
Among the different types of nanoparticles in recent years (metal oxide, metals, silica, polymers, etc.), significant scientific interest has been developed around Superparamagnetic Iron Oxide Nanoparticles (SPIONs) with a spinel structure. This is because human tissues can contain iron or iron oxide in the form of hemosiderin, ferritin, and transferrin. Normal liver contains about 0.2 mg of iron per gram, and the total human iron stores are 3,500 mg. Chronic iron toxicity is observed for an iron concentration in the liver exceeding 4 mg Fe/g [1].
Thanks to their magnetic properties, SPIONs make a very good candidate in the biomedical field, particularly as a contrast agent for MRI (Magnetic Resonance Imaging), treatments for hyperthermia, and cell imaging.
Toxicology is the study of the harmful effects of chemical, physical, biological agents on people, animals, and the environment. The toxicological study is systematic for compounds in direct contact with the human body, in the fields of diagnosis, therapy, cosmetics, and foods. Toxic cellular effects result in altered mitochondrial activity, membrane leakage, and morphological changes, which can adversely affect cell viability, proliferation, and metabolic activity and thus impair the efficiency of therapy. Toxicity tests are used to study vital cell activity such as cell death and cell viability. They are therefore essential for the development of research on nanoparticles.
Through this work, we are presenting the most used tests to evaluate the toxicity of nanoparticles, and particularly of SPIONs, in relation to cells.
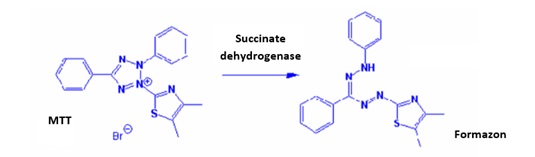
Figure 1: Formation of formazan thanks to the reduction of MTT with succinate dehydrogenase.
This test was used to detect the toxicity of iron oxide nanoparticles playing an important role in the development of chemotherapies [4,5], hyperthermia tumor treatments [6] and to evaluate the hierarchical architectures of ZnO including nanoparticles [7].
THE XTT TEST
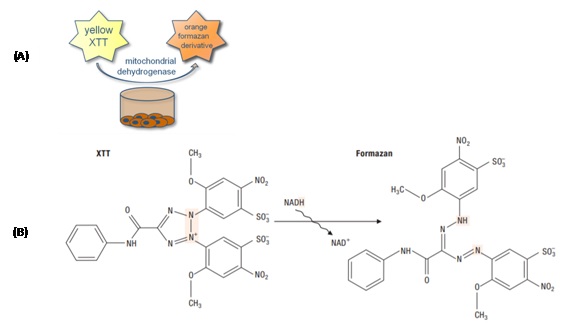
XTT or sodium (2,3-bis(2-methoxy-4-nitro-5-sulfophényl)-2H-tétrazolium-5-carboxanilide)) is a derivative of tetrazolium. Similar to MTT, XTT measures cell viability based on the activity of mitochondrial enzymes in living cells, especially under the effect of nanoparticles [8,9]. These enzymes reduce XTT and are inactivated shortly after cell death. Unlike MTT, XTT is reduced to an orange colored product that is very soluble in water (Figure 2). It thus eliminates the solubilisation step necessary with the MTT test. The amount of product generated from the reduction of XTT is proportional to the number of living cells present in the sample and can be identified by photometric spectroscopy at 475 nm3.
THE MTT TEST
MTT or 3-[4,5-(diméthylthiazol-2-yl)]-2,5-diphenyltetrazolium bromide is a tetrazolium salt used to detect reductive metabolism in cells. The mitochondria of viable cells contain a reductase enzyme, succinate dehydrogenase, which reduces a tetrazolium salt, here the yellow colored MTT, to a purple colored formazan. After reduction, the tetrazolium salts are therefore transformed into colored products (Figure 1) which can be easily quantified by colorimetric measurements. The intensity of the dyeing is proportional to the number of living cells and their metabolic activity. This formazan product must be solubilised before quantification. It is insoluble in water [2,3].
Figure 2: Principe of XTT test (A) and formation of formazan from the XTT (B).
THE TUNEL TEST
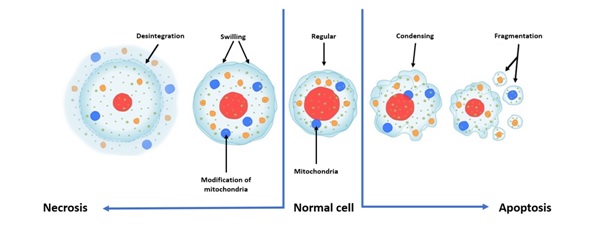
The TUNEL test is a method for analyzing DNA strand fragmentation using fluorescence microscopy. It can detect cell apoptosis. There are two major types of cell death: necrosis and apoptosis. Necrosis is the so-called “accidental” cell death that occurs during damage. The cell swells, then the cell membrane bursts, pouring the cell contents into the surrounding tissue and causing inflammation. The mitochondria swell and suffer irreversible damage. Apoptosis, also called a programmed cell death, is a physiological form of highly regulated cell death. It is necessary for the survival of multicellular organisms: cells self-destruct when they are no longer useful and/or when they are damaged or dysfunctional. It usually involves individual cells in tissue and does not cause inflammation (Figure 3).
Figure 3: Principle of necrosis and apoptosis.
This method is called “TUNEL” for Terminal deoxynucleotidyl Transferase-mediated dUTP-biotin Nick End Labeling. It consists of adding a labeled nucleotide to the free 3’-OH ends of single and double strands of DNA in order to detect fractures (single or doubles strands cuts). The test is based on the use of the terminal enzyme deoxynucleotidyl transferase (TdT) which catalyzes the attachment of deoxynucleotides to the 3’ ends of single or double strands of DNA. After permeabilization, the cells are incubated with the TUNEL reagent containing the TdT enzyme and the marker (e.g., fluorescein-dUTP). The enzyme then allows the addition of the label to the free 3’-OH groups. The label incorporated at the damaged DNA sites can be visualized, after washing, by fluorescence microscopy in the case of labeling by fluorescent nucleotides, or after a reaction, depending on the label used [2]. In studying the toxicity of magnetic nanoparticles, this test has helped to show that in vitro and in vivo toxicity results frequently contradict each other, which raises the question of protein adsorption on the surface of magnetic nanoparticles [10]. The advantage of this method is the fact of marking the cells whose morphological alterations are still difficult to visualize in optical microscopy. There are different variations of this test; however, the TUNEL technique is more sensitive and faster.
THE CYTOMETRY
Flowcytometry and laser scanning cytometry are mainly used to analyze DNA and the cell cycle. They also provide information on cell viability and cytotoxicity. Flowcytometry is a relatively old technique. Developed in the 1940s, this method is based on the evolution of the properties of cells or particles in a flow. Flowcytometry is used on the analysis of apoptosis and the cell cycle and relies on the use of fluorescent that interacts with DNA. These dyes bind to the double strands of DNA, which is mainly found in the nuclei of cells, in different ways. The most widely used are propidium iodide (PI) and DAPI (or 4’,6-diamidino-2-phenylindole dihydrochlorate) [9].
Flowcytometry allows the simultaneous measurement of several physical and biological characteristics of a cell in suspension. Initially used for the enumeration of cells in suspension and their characterization, it has become an important technique in fields such as cell proliferation, immunology, microbiology, and in the diagnosis of certain diseases. It is the only technique that allows high-speed, quantitative, multiparametric, cell by cell analysis with good detection sensitivity. These analyses require a small number of dissociated cells, simple sample preparation, and allow identification of subpopulations [2].
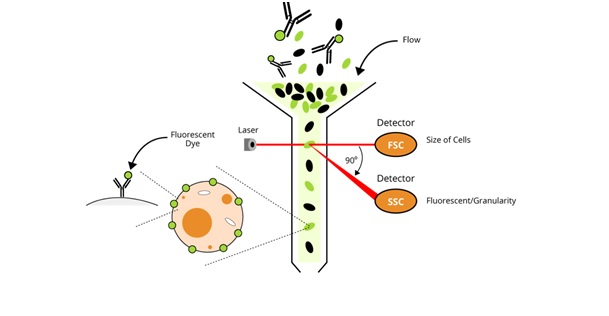
Each cell is driven by a fluid in the center of a liquid vein and then passes through a source of light excitation (usually a laser combined with lenses) used at a wavelength close to the maximum absorption of the dyes. The scattered and emitted light is then measured using a range of detectors. These measurements obtained can inform us about the size and the granularity of cells (Figure 4). In the study of the cytotoxic effects of the different hierarchical architectures of ZnO, this test made it possible to show that the nanoparticles generated greater effects on the Rat Schwann Cells (RSC) than the prism or flower-like structures [7]. However, flowcytometry involves the inability to double-check the same cell and only provides indirect data, no actual visual data, on the size or complexity of a cell or a particle.
Figure 4: Principle of flowcytometry.
Laser scanning cytometry (LSC) is based on the same principle as flowcytometry, that is, the use of fluorescent dyes to label different components of cells. Developed in the 1990s, it studies cells on the surface rather than in flow [9]. The movement of cells past the optical devices of the LSC is computerized. This makes it possible to locate cells separately and relate their different characteristics to their position. The cell cycle data obtained from an LSC is fundamentally like that provided by a flowcytometer. The addition of an optical microscope to the fluorescent apparatus made morphological studies of cells possible. This system also has a higher resolution compared to flowcytometry, which makes it possible to study the distribution pattern of a given dye in cells. Its visual capacity also offers the possibility of studying the distribution of nanoparticles in cells under a microscope. If these particles are labeled with a different fluorescent dye, this technique can simultaneously study the distribution or the number of intracellular nanoparticles and the state of the cell cycle [9].
THE COMET TEST
The Comet test can detect single and double-strand breaks, breaks at alkali-labile sites as well as indirect detection of lesions [11,12].
Principle
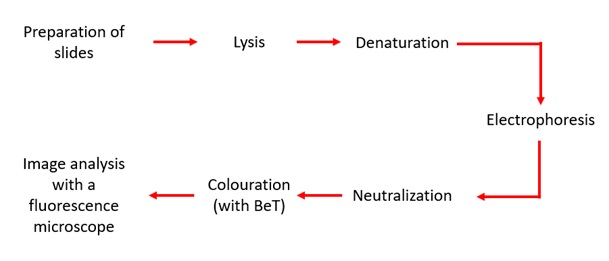
The Comet test, also called the “Single Cell Gel Electrophoresis” (SCGE) assay test in alkaline condition, is a so-called microelectrophoretic technique. It allows the in vitro and in vivo evaluation of single and double strands of DNA breaks and alkali labile sites. It can also, under certain conditions, allow the evaluation of cell repair capacities and the detection of apoptogenic products. In the nucleus, DNA molecules are in a supercoiled form. A break causing the DNA strand to break leads to a loosening of this double helix structure. This test is useful in research into the use of SPIONs for hyperthermia [6] as well as in the effect of nanoparticles on the cell cycle [9]. As shown in figure 5, the protocol allowing the visualization of these breaks follows several steps.
Figure 5: Different steps for the Comet test.
Protocol
Cell suspension: The first step of this protocol is the cell suspension which forms the basis of the test and influences its proper conduct. It is therefore important to handle the cells with care so as not to cause additional lesions. For this reason, certain steps are performed in the dark because natural light can impact cells and cause additional alkali-labile lesions.
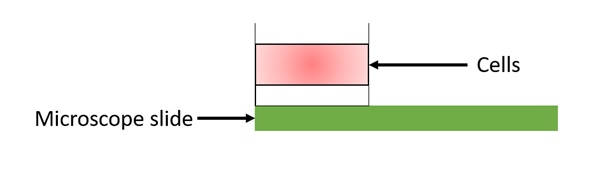
Preparation of slides: After obtaining the cell suspended in an agarose gel, it is deposited on the slide in a very specific manner illustrated in figure 6 below. It is important to choose the slide as well as the agarose gel to obtain a good image quality and to obtain optimal information on the level of DNA damages.
Figure 6: Sandwich of three layers of agarose.
Lysis: In this step, removing cell membranes, nuclear membranes, and proteins that are bound to DNA. It is this step that makes the cell-permeable. At this stage, DNA molecules still have a supercoiled structure.
Denaturation: Performed under strongly alkaline conditions, the third step involves the relaxation of the DNA molecules followed by its unwinding and by the denaturation of the double strand of DNA. The DNA molecule unwinds at its ends and at the single-strand breaks. This step is therefore essential for the detection of a single or double-strand breaks and alkali-labile sites. Using the same unwinding and electrophoresis solution balances the agarose gel with this solution before performing the last step.
Electrophoresis: When a potential difference is applied, the DNA, being negatively charged, migrates to the anode. The fragments will migrate much further due to their lightness and thus form a trail called the tail of the comet.
Neutralization and coloring: The slides are then rinsed with a buffer solution pH = 7.4, this allows the neutralization of the alkaline solution present in the gel and the elimination of salts and detergents. Otherwise, they would generate significant background noise when reading the results. Then comes the coloring step. The most widely used fluorochromes are ethidium bromide (BET) and PI, these are intercalating agents with the property of binding between DNA strands. The use of these agents results in very good quality images.
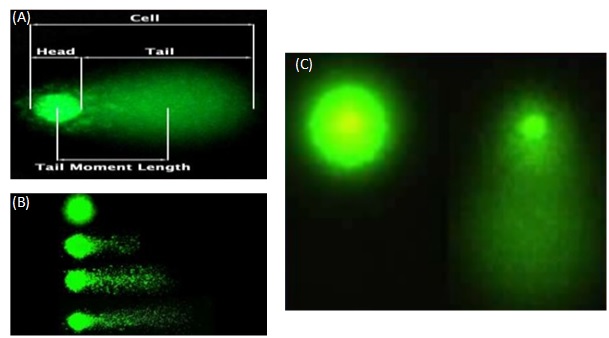
Link analysis: The slides are always read in the dark under a fluorescence microscope. The analysis can be performed with or without software. The results will then be qualitative (visual classification) or quantitative. The different types of comets are shown in figure 7.
Figure 7: Description of a Comet assay (A), different types of migration of Comet assay (B), visualization of the tail of a Comet assay (C).
CONCLUSION
There are many tests to determine the toxicity of nanoparticles. This report presents some of the most used and relevant. Nanoparticles have many properties that can be exploited in various fields. The interaction between nanoparticles and cells is a key issue in defining the toxicity of these as well as the surface coatings which differ according to use. In many articles, it is reporting that the pooling of results concerning research on nanoparticles is insufficient. Moreover, it is difficult to compare the results of all the studies because nanoparticles have different characteristics.
REFERENCES
- Corot C, Robert P, Idée JM, Port M. (2006). Recent Advances in Iron Oxide Nanocrystal Technology for Medical Imaging. Advanced Drug Delivery Reviews. 58(14):1471–504.
- Sandra C. (2008). Caractérisation de La Mort Des Cellules Animales Cultivées En Bioréacteur. Université Henri Poincaré - Nancy I. Français.
- Analyse de Biologie Cellulaire | Coloration Des Cellules | Traceurs Cellulaires. Interchim. Catalogue BioScience.
- Mahmoudi M, Sant S, Wang B, Laurent S, Sen T. (2011). Superparamagnetic Iron Oxide Nanoparticles (SPIONs): Development, Surface Modification and Applications in Chemotherapy. Advanced Drug Delivery Reviews. 63(1-2):24–46.
- Calero M, Chiappi M, Lazaro-Carillo A, Rodriguez MJ, Chichon FJ, et al. (2015). Characterization of Interaction of Magnetic Nanoparticles with Breast Cancer Cells. Journal of Nanobiotechnology. 13(1):16.
- Laurent S, Dutz S, Hafeli UO, Mahmoudi M. (2011). Magnetic Fluid Hyperthermia: Focus on Superparamagnetic Iron Oxide Nanoparticles. Advances in Colloid and Interface Science. 166(1-2):8–23.
- Yixia Y, Lin Q, Sun H, Chen D. (2012). Cytotoxic Effects of ZnO Hierarchical Architectures on RSC96 Schwann Cells. Nanoscale Research Letters. 7(1):439.
- Mahmoudi M, Saeedi-Eslami SN, Shokrgozar MA, Azadmanesh K, Hassanlou M, et al. (2012). Cell ‘Vision’: Complementary Factor of Protein Corona in Nanotoxicology. Nanoscale. 4(17):5461.
- Mahmoudi M, Azadmanesh K, Shokrgozar MA, Journeay WS, Laurent S. (2011). Effect of Nanoparticles on the Cell Life Cycle. Chemical Reviews. 111(5):3407–32.
- Markides H, El Haz AJ. (2012). Biocompatibility and Toxicity of Magnetic Nanoparticles in Regenerative Medicine. Journal of Nanomaterials. 2012:614014:1–11.
- Zennouche N. (2005). Le Test Comètes: Validation et Étude de Sa Sensibilité et Spécificité in Vitro et in Vivo. Université Henri Poincaré - Nancy I. Français.
- Sébastien L. (2004). Intérêts du test des comètes pour l’évaluation de la génotoxicité environnementale. Sciences agricoles. Université Paul Verlaine - Metz, Français.
Copyright: Fleutot S, et al. ©2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Fleutot S (2020). Nanoparticles Toxicity and Biocompatibility Tests. Nanoparticle 2(1): 06.
 Abstract
Abstract  PDF
PDF