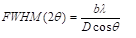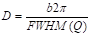Past Issues
Synthesis of Silica Nanoparticles from Sugarcane Bagasse by Sol-Gel Method
Yatim Lailun Ni’mah*, Zakkiyyah Hidayatul Muhaiminah, Suprapto Suprapto
Chemistry Department, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember Surabaya 60111, Indonesia
*Corresponding author: Yatim Lailun Ni’mah, Chemistry Department, Faculty of Science and Data Analytics, Institut Teknologi Sepuluh Nopember Surabaya 60111, Indonesia. Email: [email protected]
Received Date: June 29, 2022
Publication Date: February 16, 2023
Citation: Ni’mah YL, et al. (2023). Synthesis of Silica Nanoparticles from Sugarcane Bagasse by Sol-Gel Method. Nanoparticle. 4(1):10.
Copyright: Ni’mah YL, et al. © (2023).
ABSTRACT
The silica nanoparticles have been successfully synthesized from sugarcane bagasse using the sol-gel method. Sugarcane bagasse contains abundant silica, so it can use as an alternative source of silica nanoparticles. Sugarcane bagasse was calcined at 650°C for 2 hours, and then it was extracted using NaOH to obtain sodium silicate solution. The solution was titrated with HCl for gelling process. The synthesized of silica nanoparticles from sugarcane bagasse were characterized using FTIR and XRD. Based on the XRD characterization, the results showed that the silica had an amorphous phase, and the FTIR spectrum has siloxane and silanol groups. The synthesized silica nanoparticles have an average size of 53 nm which was calculated using Scherrer Formula. From the data obtained, sugarcane bagasse can use as a source of silica nanoparticles.
INTRODUCTION
The large amount of solid agricultural waste can be an environmental problem due to garbage accumulation, leads odor and health problem for human, including sugarcane bagasse. Sugarcane is one of the principal agricultural corp in Indonesia and it is the second largest agro-based industry after palm oil [1]. Sugarcane bagasse has been only used as animal feed, fertilizer production or reused as fuel in sugar factories and the ashes just thrown away. Chindaprasirt and Rattanasak [2] stated that sugarcane bagasse ash has a rich content of silica, where sugarcane roots play an important role in absorbing silica from the soil and transporting it to shoots, so it can used as a source of silica nanoparticle. Source of silica can found in various agricultural waste such as in sugarcane leaf and bagasse, rice husks, wheat husk, corn cob, nut grass, etc. Among many different natural resources available, sugarcane is one of the main potential source for silica extraction and subsequent transformation on nanostructured silicon [3].
Currently, nanotechnology is widely used in various important fields of science and technology such as electronics, medical, industry, etc. Development of ceramic nanoparticles with improved properties has been studied with much success in synthesis and surface science such as silica, alumina, titania, zirconia, silicon nitride, silicon carbide, etc [4]. Silica is the second most abundant element in the earth surface, which accounts for approximately 32% of the total weight of it [5]. From previous studies, silica nanoparticles are used in various application such as sensor [6], polymer filler [7], drug delivery system [8] and dye-synthesized solar cell [9].
The synthesis of oxide nanoparticles attracts more and more attention because these nanoparticles exhibit electrical, optical and magnetic properties that are different from their bulk counterparts [10]. Application of silica nanoparticles as fillers in the preparation of nanocomposite of polymers has drawn much attention, due to the increased demand for new materials with improved thermal, mechanical, physical, and chemical properties [4]. Li et al. [7] prepared solid-state electrolyte with a walnut-like silica as nano-fillers and could effectively promote the mechanical property and electrochemical performance for next generation all solid-state lithium battery. Therefore, the synthesis of silica nanoparticles needs to be considered and research improved to develop its application.
Several method has used in preparation of silica nanoparticles including hydrolysis, pyrolysis, precipitation and sol-gel method [11]. Sol-gel process is a proper route to synthesis silica nanoparticles due to its ability to form high purity and homogeneous products at proper conditions [12]. Sodium hydroxide (NaOH) is preferred for alkali-SiO2 extraction method for production of silica instead of sodium carbonate (Na2CO3), because Na2CO3 produces enormous amount of CO2 during the process of extraction and this makes the process unsustainable to the environment [13]. Sugarcane bagasse ash is one of the most rich material of silica containing about 88% to 98% silica after purification [3,12,14]. The main impurities in silica present in industrial ash are Fe2O3 and Al2O3 (19.3 and 15.6 wt %, respectively), as a result of the mechanical processes of harvesting, cutting, transporting, milling and storing bagasse, whereas in the ash prepared under controlled laboratory conditions, the main impurities are K2O and P2O5 (24.8 and 11.5% by weight, respectively) [3]. The amount of the silica content in sugarcane bagasse is varied depending on the surrounding environment, nature of soil, period of harvesting and process involve [14].
According to Vaibhav et al. [15], synthesis silica nanoparticle from sugarcane bagasse obtained the high purity of 98% with the yield of 71%. Another study reported by Falk et al. [3] stated that synthesis silica nanoparticle from sugarcane bagasse ash by sol-gel method showed an average size ~10 nm, high level of purity ~97% and predominantly amorphous character. Based on the described background, in this study sugarcane bagasse from waste of juice shop used to synthesis silica nanoparticles through sol-gel method. Chemical composition, crystalline phases and particle size were characterized.
EXPERIMENTAL
Synthesis Silica Nanoparticle from Sugarcane Bagasse
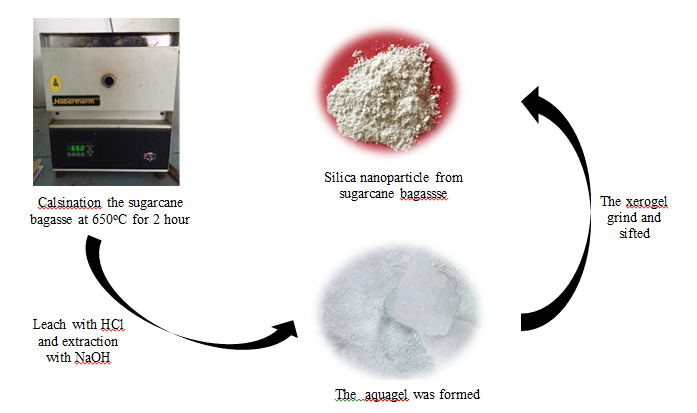
Extraction of silica nanoparticle was carried out through sol-gel method [3]. The source of sugarcane bagasse was collected from waste juice shop around Surabaya, Indonesia. The sugarcane bagasse was washed and dried then cut into smaller size. Sugarcane bagasse calcined at 650°C for 2 hours. 1 gram of sugarcane bagasse ash was leached with 10 ml HCl 1 M (HCl 37% Merck) and stirred under constant stirring for 1 h at 100°C. The leached sugarcane bagasse ash was filtered and the residue was washed with distilled water several times. The residue was added with 8 mL NaOH 1 M (Merck) and stirred under constant stirring at 100°C for 1 h. The mixture filtered and the filtrate taken, it was sodium silicate. The solution was titrated with HCl 1 M for gelling process. The aquagel formed was then left for 24 h to ageing process and washed with distilled water several times then dried. The xerogel of synthesized silica grinded in an agate mortar up to powder. The silica nanoparticle obtained, and then sifted with a 200 mesh sieve to obtain a homogeneous size of silica nanoparticle.
Scheme 1: Synthesis of silica nanoparticle from sugarcane bagasse.
Characterization
Fourier transform infrared spectroscopy (FTIR) was carried out to characterize the silica nanoparticles from sugarcane bagasse. An FTIR spectrum was acquired with a Shimadzu FTIR-8400 spectrometer over a wavenumber range from 400-4000 cm-1 at room temperature [16].
The crystalline phase of the synthesized silica nanoparticle was determined by X-Ray diffraction (XRD) using Philips X-Pert XRD with a Cu-Kα light source (λ = 1.5405Å, 40 kV and 30 mA). Samples were analyzed in the range of 2θ = 5o-60o with a scan speed of 0.5o/min at room temperature [17]. The crystallite size estimated using Scherrer Formula (1) and (2):
Where FWHM is the full width of the peak, λ is the wavelength, Q is the magnitude of the scattering vector, b is a constant, which normally takes a value between 0.89 and 0.94 depending on the function used to fit the peak and D is the dimension of the crystallites monodisperse in size [17].
RESULT AND DISCUSSION
Silica nanoparticle was produced from sugarcane bagasse ash using sol-gel method. The chemical composition of synthesized silica is shown in Figure 1 represents FTIR spectra of silica nanoparticle from sugarcane bagasse. The adsorption bands at 3421 cm-1 is due to stretching vibration of the –OH bond, whereas peak at 1636 cm-1 is attributed to bending vibration of H2O from silanol group (Si-OH) [13]. The bands at 1050 cm-1 are assigned to Si-O-Si asymmetric stretching vibration and peak at 793 cm-1 shows symmetric stretching vibration of Si-O-Si bond [18]. The peak at 436 cm-1 is related to bending vibration of Si-O-Si bond. The solubilization of sodium silicate with hydrochloric acid leads the formation of silanol groups, while siloxane groups are formed due to condensation [19]. The FTIR spectra clearly indicating the formation of silica synthesized from sugarcane bagasse well formed.
Figure 2 represents the XRD pattern of silica nanoparticle from sugarcane bagasse ash which can be used to qualitatively and quantitatively analyze the degree crystalline. Falk et al., [3] stated that the crystalline peaks observed in sugarcane bagasse ash related to SiO2 in the form of quartz (JCPDS 01-085-0796), Al2O3 (JCPDS 01-0851337), and Fe2O3 (JCPDS 01-087-1164). The characteristic peak corresponding to silica observed at 2θ of 22.6o. Based on the peak at the XRD pattern, it showed a wide peak indicate to amorphous phase of silica [20]. The crystallite size estimated by the Scherrer formula based on the peak at 2θ of 22.6o was 53 nm.
Figure 1: FTIR spectra of silica nanoparticle from sugarcane bagasse using sol-gel method.
Figure 2: XRD pattern of silica nanoparticle from sugarcane bagasse using sol-gel method.
CONCLUSION
The silica nanoparticle has been successfully synthesized from sugarcane bagasse through sol-gel method. This method is simple and effective for synthesis silica nanomaterial from sugarcane bagasse. From XRD spectra, silica nanoparticle was an amorphous phase and has average size is 53 nm.
- REFERENCES
- Iryani D, Hirajima T, Kumagai S, Nonaka M, Sasaki K. (2012). Overview of Indonesian Sugarcane Industry and Utilization of Its Solid Waste.
- Chindaprasirt P, Rattanasak U. (2020). Eco-production of silica from sugarcane bagasse ash for use as a photochromic pigment filler. Sci Rep 10:9890.
- Falk G, Shinhe GP, Teixeira LB, Moraes EG, de Oliveira APN. (2019). Synthesis of silica nanoparticles from sugarcane bagasse ash and nano-silicon via magnesiothermic reactions. Ceramics Int. 45:21618-21624.
- Ab Rahman I, Padavettan V. (2012). Synthesis of Silica Nanoparticles by Sol-Gel: Size-Dependent Properties, Surface Modification, and Applications in Silica-Polymer Nanocomposites—A Review. J Nanomater. 2012.
- Ghorbani F, Younesi H, Mehraban Z, Çelik MS, Ghoreyshi AA, Anbia M. (2013). Preparation and characterization of highly pure silica from sedge as agricultural waste and its utilization in the synthesis of mesoporous silica MCM-41. J Taiwan Institute Chem Engineers. 44:821-828.
- Bonyadi S, Ghanbari KH. (2021). Development of highly sensitive and selective sensor based on molecular imprinted polydopamine-coated silica nanoparticles for electrochemical determination of sunset yellow. Microchem J. 167:106322.
- Li X, Yang L, Shao D, Lu K, Liu L, Wu Z, et al. (2020). Preparation and application of poly(ethylene oxide)‐based all solid‐state electrolyte with a walnut‐like SiO2 as nano‐fillers. J Appl Polymer Sci. 137.
- Porrang S, Rahemi N, Davaran S, Mahdavi M, Hassanzadeh B, Gholipour AM. (2021). Direct surface modification of mesoporous silica nanoparticles by DBD plasma as a green approach to prepare dual-responsive drug delivery system, Journal of the Taiwan Institute of Chemical Engineers.
- Fang Y, Zhang J, Zhou X, Lin Y, Fang S. (2012). Soggy sand’ electrolyte based on COOH-functionalized silica nanoparticles for dye-sensitized solar cells. Electrochem Communications. 16:10-13.
- Xu H, Wang X. Zhang L. (2008). Selective preparation of nanorods and micro-octahedrons of Fe2O3 and their catalytic performances for thermal decomposition of ammonium perchlorate. Powder Technol. 185:176-180.
- Adebisi JA, Agunsoye JO, Bello SA, Ahmed II, Ojo OA, Hassan SB. (2017). Potential of producing solar grade silicon nanoparticles from selected agro-wastes: A review. Solar Energy. 142:68-86.
- Boonmee A. Jarukumjorn K. (2020). Preparation and characterization of silica nanoparticles from sugarcane bagasse ash for using as a filler in natural rubber composites. Polymer Bulletin. 77:3457-3472
- Nayak PP, Datta AK. (2020). Synthesis of SiO2-Nanoparticles from Rice Husk Ash and its Comparison with Commercial Amorphous Silica through Material Characterization. Silicon. 13:1209-1214.
- Norsuraya S, Fazlena H, Norhasyimi R. (2016). Sugarcane Bagasse as a Renewable Source of Silica to Synthesize Santa Barbara Amorphous-15 (SBA-15), Procedia Engineering. 148:839-846.
- Vaibhav V, Vijayalakshmi U, Roopan SM. (2015). Agricultural waste as a source for the production of silica nanoparticles, Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 139:515-520.
- Ji KS. Moon HS. Kim JW, Park JW. (2003). Role of functional nano-sized inorganic fillers in poly(ethylene) oxide-based polymer electrolytes. J Power Sources. 117:124-130.
- Ingham B, Toney MF. (2014). 1 - X-ray diffraction for characterizing metallic films, in Metallic Films for Electronic, Optical and Magnetic Applications. Barmak K, Coffey K, Eds. Woodhead Publishing: 3-38.
- Premaratne WAPJ, Priyadarshana WMJI, Gunawardena SHP, de Alwis AAP. (2013). Synthesis of Nanosilica from Paddy Husk Ash and Their Surface Functionalization. J Sci Univ Kelaniya. 8:33-48.
- Azat S, Korobeinyk A, Moustakas K, Inglezakis V. (2019). Sustainable production of pure silica from rice husk waste in Kazakhstan. J Cleaner Product. 217:352-359.
- Le VH, Thuc CNH, Thuc HH. (2013). Synthesis of silica nanoparticles from Vietnamese rice husk by sol–gel method, Nano Res Letters. 8:58.
 Abstract
Abstract  PDF
PDF