Past Issues
Reproductive Toxicity of Aluminum Oxide Nanoparticles and Zinc Oxide Nanoparticles in Male Rats
Mokhtar I. Yousef a,*, Mohammed Y.I. Al-Hamadani a, Maher A. Kamel b
a Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, Alexandria, Egypt. b Department of Biochemistry, Medical Research Institute, Alexandria University, Alexandria, Egypt.
*Corresponding author: Mokhtar I. Yousef, Department of Environmental Studies, Institute of Graduate Studies and Research, Alexandria University, 163 Horreya Avenue, Chatby 21526, P.O. Box 832, Alexandria, Egypt.
Received : November 11, 2019 Published : December 13, 2019
ABSTRACT
The rapid development of nanotechnology raises both interest and concern among researchers. Thus, this study investigated the reproductive toxicity of aluminum oxide nanoparticles (Al2O3NPs) and zinc oxide nanoparticles (ZnONPs) alone or their combination in male rats via testicular damage, spermatoxicity, DNA fragmentation, mitochondrial transcription factor A (mtTFA) gene, uncoupling protein 2 (UCP2) gene, tumor suppressor gene p53 (p53), cytokines, oxidative stress, sex hormones and testes histopathology. Animals were administered orally with Al2O3NPs (70 mg/kg BW), ZnONPs (100 mg/kg BW) or their combination every day for 75 days. The obtained results represented that exposure to Al2O3NPs and ZnONPs or their combination caused marked DNA fragmentation and at gene expression level mtTFA gene was significantly suppressed. Semen characteristics, testosterone, TSH, antioxidant enzymes and reduced glutathione were significantly decreased. Whereas, free radicals, nitric oxide, UCP2 gene, p53, tumor necrosis factor-α, interliukin-6, FSH, LH, T3 and T4 were significantly increased. Moreover, testes histopathological examination and steroidogenesis enzymes were changed. In conclusion, the present study showed that the reproductive toxicity induced by the combination of Al2O3NPs with ZnONPs was more pronounced than each one.
Keywords: Aluminum oxide nanoparticles; Zinc oxide nanoparticles; Male rats; Reproductive toxicity; Mitochondrial transcription factor A; Uncoupling protein 2; DNA fragmentation; Semen characteristics; hormones; Oxidative stress; Antioxidants; Cytokines; Histopathological changes
INTRODUCTION
Among various nanoparticles (NPs), aluminum oxide nanoparticles (Al2O3NPs) are one of the most important because of its promising technological applications. It is currently one of the two US market leaders for nanosized materials [1]. Based on these extensive commercial applications, they have drawn considerable attention as potential environmental pollutants [2]. The genotoxicity observed with Al2O3NPs may be due to pro-inflammatory effects through a reactive oxygen species (ROS)-mediated mechanism [3]. Prabhakar et al. [4] demonstrated significant increase in myocardial malondialdehyde with significant reduction in myocardial SOD and CAT enzymes activities and GSH concentration in nano-alumina intoxicated rats suggesting enhanced oxidative stress state in myocardium. Some articles reported micronuclei increase [5], DNA damage [5] and chromosomal aberrations [6] caused by AlCl3NPs.
Also, zinc oxide nanoparticles (ZnONPs) are among the metal oxide nanoparticles and have received considerable attention. This is due to their low production cost, ability to form diverse structures and various biological applications including drug delivery, bio-imaging probes, cancer treatment, antibacterial and immunomodulatory agent [7]. Previous studies have shown the cytotoxic and pro-inflammatory effects of ZnONPs in different types of cells [8]. In vitro and in vivo studies have shown that ZnO nanoparticles caused membrane damage, inflammation, DNA damage, apoptosis and complex cell-cell and cell- matrix interactions and changes in some hormones in mammalian cells [9]. Adverse effects of ZnONPs can be mainly attributed to the generation of ROS leading to membrane damage, directly or indirectly [10]. There is scarce information about the possible reproductive toxicity of the aluminum oxide nanoparticles (Al2O3NPs) and zinc oxide nanoparticles (ZnONPs) alone or in combination despite of their current use in novel technology. Therefore, the potential risks on reproductive health should be investigated, especially in those who are occupationally exposed to Al2O3NPs and ZnONPs. To achieve this aim, we determined semen characteristics, DNA fragmentation, mitochondrial transcription factor A gene, uncoupling protein 2 gene, p53, cytokines, oxidative stress and sex hormones and testes histopathology in male rats.
MATERIALS AND METHODS
Tested compounds and doses
Al2O3NPs nanopowder (about 50 nm particle size) and ZnONPs nanopowder (about 100 nm particle size), were purchased from Sigma Aldrich Chemical Company (St. Louis, MO, USA). The dose of aluminum oxide nanoparticles was 70 mg/kg BW (aqueous suspension) and was chosen according to [11]. The dose of ZnONPs was 100 mg/kg BW (aqueous suspension) and was chosen [12].
Animals and experimental groups
Forty Wistar male albino rats 4-5 months age and weighing 160-170g were used in the present study. Animals were housed in a stainless steel wire cages and given food and water ad libitum. Animals were maintained in a controlled atmosphere, a temperature of 25 ± 5 °C and 50-70% humidity. After two weeks of acclimation, animals were divided into four equal groups as follows: a control group and 3 treated groups; group 2, 3 and 4 which were orally treated with Al2O3NPs (70 mg/kg BW), ZnONPs (100 mg/kg) and Al2O3NPs plus ZnONPs, respectively. Rats were orally administered their respective doses daily for 75 consecutive days.
Blood samples collection and tissue preparations
At the end of the 75th day of the experimental period, all animals of each group were anaesthetized with diethyl ether and sacrificed. Blood samples were collected by cardiac puncture from anaesthetized rats in test tubes containing heparin as an anticoagulant. Blood samples were centrifuged at 860 ×g for 20 min for the separation of plasma. Plasma was kept at - 80 °C until analyses of the tested parameters. Testes were immediately removed, washed using chilled saline solution (0.9%), and removed the adhering fat and connective tissues. testes were minced and homogenized (10%, w/v), separately, in ice-cold sucrose buffer (0.25 M) in a Potter–Elvehjem type homogenizer. The homogenates were centrifuged at 10,000 ×g for 20 min at 4 °C, to pellet the cell debris and the supernatant was stored at −80 °C for the determination of tested parameters.
Body and organs weights
Initial and final body weights of male rats were recorded and subsequently body weight gain (g/75 days) was calculated. Body weight gain (g/75 days) = Final weight – Initial weight. The reproductive sex organs (testes, prostates and epididymis) were immediately removed, washed using chilled saline solution (0.9%), removed the adhering fat and connective tissues, dried on tissue papers and weighed.
Quantitative analysis of testicular gene expression of mitochondrial transcription factor A (mtTFA) and uncoupling protein 2 (UCP2) using quantitative real time reverse transcriptase- polymerase chain reaction (qRT-PCR)
Total RNA was isolated from testicular tissues using GF-1 Total RNA Extraction Kit (Vivantis, Malaysia) ViP rime PLUS One step Quantitative Real-Time Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR) Green Master Mix (Vivantis, Malaysia) was used for the relative quantitative determination of the gene expression of mtTFA [13] and UCP2 [14] at mRNA level using GAPDH as an internal reference gene for validation of the extraction procedure and calculation of relative expression.
Assay of DNA fragmentation as a marker of cell death
Total DNA in the tissues was isolated using DNeasy kit (Qiagen, Germany) and the concentration and purity DNA were assessed using Nanodrop2000@ (Thermo Fisher Scientific, USA). Then DNA fragmentation, as a marker of cell death, was assayed [15].
ELISA measurements
Tumor suppressor gene p53, tumor necrosis factor-alpha (TNF-a) and interleukin-6 (IL-6) were assayed using Enzyme-linked Immunosorbent Assay (ELISA) kitsin testes tissue homogenates according to the manufacturer instructions using the serial standard of each parameter.
Markers of oxidative stress
The process of lipid peroxidation resulted in the end product of malondialdehyde (MDA). The MDA was determined as thiobarbituric acid-reactive substances (TBARS) assay in which MDA was heated with thiobarbituric acid (TBA) at a low pH to produce a pink chromogen with a maximum absorbance at 532 nm. The level of TBARS was calculated from a standard curve constructed using serial concentration of tetramethoxypropane (TMP) [16]. The Griess reaction was used to determine the concentration of nitrite and nitrate as nitric oxide end products (NOx) in the deproteinized samples. The Griess reaction was supplemented with the reduction of nitrate to nitrite by NADPH-dependent nitrate reductase. The assay procedure consisted of two steps: the first required the diazotization of sulphanilic acid with nitrite ions followed by the second step of coupling this product with a diamine, resulting in a measurable pink metabolite 540 nm. The level of NOx was determined from the slope of the standard curve constructed using serial concentration of sodium nitrite. The total antioxidant capacity (TAC) and the activities of superoxide dismutase (SOD), glutathione peroxidase (GPX), glutathione S-transferase (GST) and catalase (CAT) in the tissue homogenates were measured using colorimetric kits (Biodiagnostic, Egypt) according to the manufacturer instructions and using specific standard for each parameter. Reduced glutathione content was assayed after protein precipitation using a metaphosphoric acid reagent. The assay was based on the oxidation of GSH by 5,5′-dithiobis-(2-nitrobenzoic acid) (DTNB) to yield GSSG and 5-thio-2-nitrobenzoic acid (TNB). The rate of TNB formation was assayed at 412 nm and was proportional to the GSH present in the sample. The rate of formation of TNB was monitored by recording the change in the absorbance. This was found to be 412 nm per minute (ΔA/min). The total glutathione content in the samples was determined from a GSH standard curve and the results were subsequently expressed as nmol/g tissue by dividing the concentration of glutathione in the sample by the weight (in grams) of tissue used to prepare the sample [17].
Determination of 17-ketosteroid reductase and 17β-hydroxysteroid dehydrogenase activities in testes
Streoidgenic 17-ketosteroid reductase enzyme activity (17-KSR; EC 1.1.1.64) and 17β–hydroxysteroid dehydrogenase enzyme activity (17β-HSD; EC 1.1.1.51) were estimated [18].
Semen characteristics
The left epididymis was excised and placed in a warmed petri dish contain 0.2 ml of calcium and magnesium free Hank’s solution at 37 °C. The tissue was minced with scalpels for approximately 1 min and placed in a 37 °C incubator for 15 min prior to determining sperm motility. The suspension was stirred, one drop was placed on a warmed microscope slide, and a 22x22 mm coverslip was placed over the droplet. At least 10 microscopic fields were observed at 400 x magnification using a phase-contrast microscope, and the percentage of motile sperm was recorded [19,20]. The coverslip was removed and the spermatozoa suspension was allowed to dry in air. The sample was stained with 1% eosin Y/5% nigrosin and examined at 400 x for morphological abnormalities. Three hundred spermatozoa from different fields were examined for each sample as described previously [19,20].
The right epididymis and specimens of right testis were frozen immediately after weighing. After thawing at room temperature, the whole epididymis and specimens of testis were homogenized in 0.5 ml of a solution of 0.9% NaCl containing 2% of Triton X-100. Ten strokes of a manual glass homogenizer were used for each sample. The testis and epididymis homogenates were diluted with 1.5 ml of the homogenization solution and spermatozoa were counted at 400 x magnification using a Neubauerhemocytometer. Three counts per sample were averaged [20]. Assessment of sperm count and abnormal spermatozoa was performed using an eosin-nigrosine blue staining mixture as described by Blom [21].
Determination of reproductive and thyroid hormones
Testosterone was determined by using Enzyme-linked Immunosorbent Assay (ELISA) kit for the quantitative measurement of testosterone in plasma (DRG international co., U.S.A). Follicle stimulating hormone (FSH) was assayed by using ELISA kit for the determination of Follitropin in rat plasma (BIOCODE-HYCEL co. Rue E. Solvay, 101-103 - 4000 Liège - Belgium). Luteinizing hormone (LH) was determined by using ELISA kit for the quantitative determination of Rat LH concentrations in serum, plasma and other biological fluids (Elabscience Biotechnology Co., Ltd). Rat thyroid stimulating hormone (TSH) was assayed by using ELISA kit for the quantitative determination of rat TSH concentrations in plasma (CUSABIO BIOTECH CO., LTD.). Triiodothyronine (T3) was assayed by using ELISA Kit for the quantitative measurement of T3 in rat plasma (Abnova CO., KA 0925). Rat Thyroxine (T4) was assayed by using ELISA kit in vitro quantitative detection of rat in plasma (Biovision CO., U.S.A).
Histological section preparation of testes
Testes was obtained from rats, and immediately fixed in 10% formalin, and then treated with a conventional grade of alcohol and xylol, embedded in paraffin and sectioned at 4-6 μm thickness. The sections were stained with Haematoxylin and Eosin (H&E) stains and photographed on the PC screen using a light microscope (Olympus BH-2; Japan) with a digital color camera attachment (Sanyo VVC-6975 P; Japan) and dial indicator for studying the histopathological changes [22].
Statistical analysis
Results are reported as means ± SE. Statistical analysis for all studied parameters was performed using the general linear model (GLM) produced by Statistical Analysis Systems Institute [23]. Duncan's New Multiple Range Test was used to test the significance of the differences between means [24]. Values of p<0.05 were considered statistically significant.
RESULTS
Initial and final body and sex organ weights
Data showed that there is no significant difference between control and all treated groups in the initial body weight. While, final body weight, body weight gain, testes and epididymis weights significantly decreased, and prostates weight significantly increased in aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination compared to control group. The final body weight, body weight gain, epididymis and prostates weights showed a similar pattern of change between Al2O3NPs and ZnONPs groups. It was pronounced that the effect of the combination was more than each of them alone (Table 1).
Table 1: The body and organs weights of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination.
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
I.B.W. (g) |
169±3.17 |
167±2.38 |
170±2.24 |
168±2.91 |
|
F.B.W. (g) |
222±6.16a |
207±3.67b |
201±5.50b |
195±4.53b |
|
B.W.G (g/75 days) |
53.5±6.54a |
40.0±5.37ab |
30.5±5.40b |
27.0±5.12b |
|
Testes weight (g) |
2.78±0.06a |
2.55±0.07b |
2.14±0.10c |
2.09±0.07c |
|
Epididymis weight (g) |
2.85± 0.08a |
2.29± 0.06b |
2.20± 0.10bc |
1.96± 0.10c |
|
Prostate weight (g) |
0.59±0.03a |
0.65±0.02ab |
0.70±0.03b |
0.71±0.03b |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. (IBW = Initial body weight, FBW = Final body weight, BWG= Body weight gain).
Testicular gene expression of mitochondrial transcription factor-A (mtTFA) and uncoupling protein 2
Data showed suppression of the testicular gene expression of mitochondrial transcription factor-A (mtTFA) in testes of rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination by about 50%, 45% and 59% of control value, respectively (Table 2). The testicular gene expression of uncoupling protein 2 (UCP2) showed significant induction in the rats treated with Al2O3NPs, ZnONPs and their combination by about 193%, 130% and 317% of control value, respectively (Table 2). The treatment with the combination of Al2O3NPs plus ZnONPs showed the highest induction (more 3 folds than the control level).
Table 2: The effect of aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination on the testicular gene expression of mitochondrial transcription factor-A (mtTFA) and and uncoupling protein 2 (UCP2)
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs + ZnONPs |
|
|
mtTFA |
1.0± 0.05a |
0.5± 0.17b (-50 %) |
0.55 ± 0.02b (-45 %) |
0.41 ± 0.02c (-59 %) |
|
UCP2 |
1.0± 0.06a |
2.93 ± 0.29b (193 %) |
2.30 ± 0.27b (130 %) |
4.17 ± 0.18c (317 %) |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. (Numbers between parentheses represent the percentage change from control value).
DNA Fragmentation
The agarose gel electrophoresis of testicular genomic DNA showed very low or undetectable DNA laddering (DNA fragmentation) in the testes of the control rats (Fig. 1). The DNA intact band appears to be condensed near the application point with no DNA smearing suggesting no DNA fragmentation. On the other hand, the ZnO and Al2O3 nanoparticles-treatment alone or in combination resulted in massive DNA fragmentations which appear as DNA laddering (smearing).
Tissues levels of p53, TNF-α and IL-6
The rats treated with Al2O3NPs and ZnONPs alone or in combination showed significantly higher testes level of p53, TNF-α and IL-6 compared to control group. In addition, treatment with Al2O3NPs and ZnONPs in combination group showed significant increase in the level of p53, TNF-α and IL-6 compared to Al2O3NPs and ZnONPs groups (Table 3).
Table 3: Testes levels of tumor suppressor p53 (p53), tumor necrosis factor-α (TNF-α) and interliukin-6 (IL-6) of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
p53 (ng/mg protein) |
6.10± 0.40d |
10.50± 0.40c |
11.98± 0.20b |
13.06± 0.14a |
|
TNF-α(ng/g mtissue) |
139± 1.22d |
331± 0.97b |
317± 3.56c |
401± 0.88a |
|
IL-6 (ng/gm tissue) |
117± 0.88d |
195± 1.45b |
168± 2.85c |
267± 3.93a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. P53= Tumor suppressor, TNF-α = Tumor necrosis factor-α, IL-6 = Interliukin-6
Semen characteristics and sex hormones
Data showed that Al2O3NPs, ZnONPs and their combination caused significant reduction in sperm motility and sperm count, and significant induction in abnormal sperm compared to the control group (Table 4). Data showed significant decrease in the levels of testosterone and thyroid-Stimulating hormone (TSH), and significant increase in follicle stimulating hormone (FSH), luteinizing hormone (LH), tri-Iodothyronine (T3) and Thyroxin (T4) levels in rats treated with Al2O3NPs, ZnONPs and their combination compared to the control group (Table 5).
Table 4: Semen characteristics of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
Sperm count (million/ml) |
83.0±2.56a |
30.4±1.73c |
42.3±2.18b |
25.20±1.40c |
|
Abnormal (%) |
14.2±0.86c |
36.0±1.77b |
35.4±1.87b |
42.0±2.13a |
|
Motility (%) |
87.2± 3.11a |
41.6± 2.16b |
39.6± 2.83b |
28.4± 0.68c |
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
Testosterone (ng/ml) |
5.1±0.29a |
2.1±0.08b |
2.6±0.14b |
1.3±0.05c |
|
FSH (ng/ml) |
0.69±0.04a |
1.51±0.03b |
1.59±0.05b |
1.91±0.06c |
|
LH (ng/ml) |
0.45± 0.03a |
0.94± 0.02b |
0.95± 0.04b |
1.30± 0.06c |
|
TSH (ng/ml) |
4.10± 0.23a |
1.92± 0.06b |
1.66± 0.10b |
1.04± 0.06c |
|
T3 (ng/ml) |
84.2±6.8c |
152.8±5.4b |
133.8±8.8b |
178.0±4.9a |
|
T4 (ng/ml) |
0.93±0.06c |
1.59±0.10b |
1.89±0.10b |
2.25±0.16a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05.
Antioxidant enzymes and free radicals
Treatment with Al2O3NPs, ZnONPs and their combination caused significant decrease in the activities of plasma and testes GPX, GST, CAT, SOD, GSH and TAC, and significant increase in TBARS and NO compared to control group. The effect of the combination of Al2O3NPs plus ZnONPs was more toxic than each other (Tables 6-9).
Table 6: Plasma antioxidant enzymes of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
GPX (U/ml) |
32.2±0.78a |
26.9±1.85b |
24.4±1.11b |
20.5±0.68c |
|
GST (μmole /hr) |
0.64±0.04a |
0.42±0.02b |
0.36±0.01bc |
0.29±0.02c |
|
CAT (U/ml) |
62.2±1.42a |
42.6±1.39b |
38.1±1.02c |
32.6±1.71d |
|
SOD (U/ml) |
1.23±0.05a |
0.87±0.04b |
0.93±0.02b |
0.55±0.02c |
|
TAC (mM/L) |
1.81±0.01a |
1.39±0.07b |
1.45±0.04b |
0.96±0.01c |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. GPX=glutathione peroxidase, GST=glutathione S-transferase, CAT=catalase, SOD=superoxide dismutase, TAC= total antioxidant capacity
Table 7: Plasma reduced glutathione and free radicals of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
GSH (nmol/mg) |
4.8±0.11a |
3.9±0.10b |
3.7±0.09b |
2.9±0.06c |
|
TBARS (nmol/mg) |
0.92±0.03c |
1.34±0.07b |
1.26±0.08b |
1.61±0.10a |
|
NO (u mol/L) |
24.6±0.81c |
44.1±1.67b |
45.5±1.84b |
54.1±2.71a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. GSH = reduced glutathione, TBARS = thiobarbituric acid-reactive substances, NO= nitric oxide
Table 8: Testes glutathione peroxidase, glutathione S-transferase, catalase, superoxide dismutase and total antioxidant capacity of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
GPX (U/g) |
17.3±1.08a |
13.0±0.46b |
12.3±0.54b |
5.5±0.28c |
|
GST (μmole /hr) |
2.04±0.03a |
1.69±0.04b |
1.74±0.03b |
1.32±0.08c |
|
CAT (U/g) |
54.3±0.72a |
43.8±1.91b |
43.1±2.04b |
29.6±2.35c |
|
SOD (U/g) |
13.3±0.25a |
9.7±0.33b |
9.3±0.64b |
6.7±0.46cc |
|
TAC (mM/L) |
1.7 ±0.04a |
1.2 ±0.04b |
1.3 ±0.06b |
0.9 ±0.07c |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. GPX=glutathione peroxidase, GST=glutathione S-transferase, CAT=catalase, SOD=superoxide dismutase, TAC= total antioxidant capacity
Table 9: Testes reduced glutathione and free radicals of male rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
GSH (u mol/g) |
5.0±0.17a |
4.1±0.28b |
3.9±0.29bc |
3.3±0.21c |
|
TBARS (n mol/g) |
35.1±1.53c |
50.9±0.46b |
52.7±0.99b |
59.1±2.33a |
|
NO (umol/L) |
52.6 ±1.65c |
75.2 ±3.94b |
81.2 ±2.11b |
100.5 ±1.56a |
Data presented as Mean±SE. For each parameter, the groups with different superscript letters are significantly differ, p<0.05. GSH= reduced glutathione, TBARS= thiobarbituric acid-reactive substances, NO= nitric oxide
Activities of steroidogenic enzymes
Data showed that treatment with Al2O3NPs, ZnONPs and their combination caused significant (P<0.05) increase in steroidogenic enzymes 17β-hydroxysteroid dehydrogenase (17β-HSD) activity and significant decrease in 17-ketosteroid reductase (17-KSR) comparison with the control group (Table 10).
Table 10: Testicular 17β-hydroxysteroid dehydrogenase (17β-HSD) and 17-ketosteroid reductase (17-KSR) of rats treated with aluminum oxide nanoparticles (Al2O3NPs), zinc oxide nanoparticles (ZnONPs) and their combination
|
Parameter |
Experimental groups |
|||
|
Control |
Al2O3NPs |
ZnONPs |
Al2O3NPs+ ZnONPs |
|
|
17β-HSD (U/min/mg) |
0.85±0.04c |
1.01±0.06bc |
1.13±0.03b |
1.44±0.09a |
|
17-KSR (U/min/mg) |
16.4±0.69a |
12.7±0.60b |
10.3±0.51c |
4.5±0.31d |
Histopathology
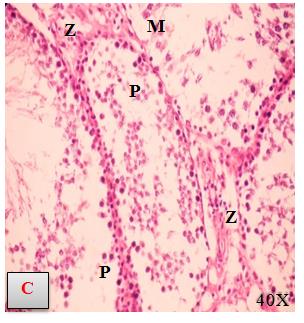
Histological examination of adult male rat testicular tissues of the different studied groups. Rat testes of control group showed regular cross sections of seminiferous tubules with clusters of interstitial cells of Leydig in between. The tubules are lined by regular stratified layers of spermatogenic cells with different stages of spermatogenesis, including spermatogonia, spermatocytes and spermatids. Spermatozoa are seen filling the Lumina of the tubules. Sertoli cells are seen resting on the basement membrane (Figure 2a and 2b). Figure 3a and 3b showed the changes in rats treated with Al2O3NPs for which showed of Al2O3NPs treated rat testis showing distorted seminiferous tubules with loosely arranged detached spermatogenic cells, Most of the spermatogenic cells are with pyknotic nuclei and falling into the seminiferous tubules lumina. Notice the wide interstitial spaces in between the tubules containing few darkly stained Leydig cells. Figure 4a and b showed the changes in rats treated with ZnONPs which showed of ZnONPs treated rat testis showing seminiferous tubules with irregular shapes and wide empty lumina. Decrease height of the lining epithelium and many spermatogenic cells with pyknotic nuclei are noticed. The Leydig cells are almost absent with wide interstitial spaces. The changes in testes histology of rats treated with Al2O3NPs plus ZnONPs were presented in Figure 5a and b which showed that testis of treated rats showed many severely distorted seminiferous tubules with disruption and irregularity of their basement membrane. Other tubules are illustrating focal losses of the germinal epithelium and the disturbed organization of the spermatogenic cells Notice the wide interstitial spaces in between some tubules while others are showing obliterated spaces containing few darkly stained Leydig cells.
Figure 1: Stained agarose gel of testicular genomic DNA demonstrating apoptotic and necrotic cell deaths induced nanoparticles. lane 1 and 2: control, lane 3 and 4: ZnO nanoparticles treated rats, lane 5 and 6: Al2O3 nanoparticles treated rats, lanes 7 and 8: ZnO plus Al2O3 treated rats.
Figure 2 A, B: Light micrographs of testis of control group which showed regular cross sections of seminiferous tubules (T) with clusters of interstitial cells of Leydig (L) in between. The tubules are lined by regular stratified layers of spermatogenic cells with different stages of spermatogenesis, including spermatogonia (Sg), spermatocytes (Sp) and spermatids (Sd). Spermatozoa (Sz) are seen filling the Lumina of the tubules. Sertoli cells (St) are seen resting on the basement membrane (B).
Figure 3 A, B: Light micrographs of testis of rates treated with Al2O3NPs which showed distorted seminiferous tubules with loosely arranged detached spermatogenic cells (R), Most of the spermatogenic cells are with pyknotic nuclei (X) and falling into the seminiferous tubules lumina. Notice the wide interstitial spaces in between the tubules containing few darkly stained Leydig cells (G).
Figure 5 A, B, C: Light micrographs of testis of the combination of Al2O3NPs with ZnO3NPs which showed many severely distorted seminiferous tubules (M) with disruption and irregularity of their basement membrane (W). Other tubules are illustrating focal losses of the germinal epithelium and the disturbed organization of the spermatogenic cells (P) Notice the wide interstitial spaces in between some tubules (G) while others are showing obliterated spaces containing few darkly stained Leydig cells (Z).
DISCUSSION
Fernandez et al. [25] found that determination of body weight supplies significant information on possible implications for the health of the organism and the toxicity of a compound. Also, the determination of absolute and relative weights of testes, epididymis, seminal vesicle and prostate can be utilized in the evaluation of risks from adverse effects on male reproductive apparatus. Body and organs weights are sensitive indicators of toxic chemicals and this is in agreement with the current study showed that exposure to Al2O3NPs, ZnONPs and their combination caused alterations in the body and sex organs weights. The effects of these nanoparticles may be referring to their toxicity on body and sex organs weights. The present study showed deterioration in sperm characteristics after exposure to Al2O3NPs, ZnONPs and their combination. Also, Sullivan et al. [26] reported that reduction in the number of spermatids in the testis and per gram testis and in daily sperm production was found.
Mitochondrial transcription factor A (mtTFA) is a key transcription factor. During spermatogenesis in the mouse, mtTFA is expressed up to the late spermatocyte and early spermatid stage [27]. Therefore, it is important to determine mtTFA in the present study to know if the tested nanoparticles have effects on mitochondrial transcription factor A (mtTFA), as a transcription factor is a multi-functional protein plays an important role in the maintenance of mitochondrial DNA (mtDNA) integrity, replication and transcription [28]. The obtained data showed that Al2O3NPs, ZnONPs and their combination caused significant decline in mtTFA by 50%, 45% and 59%, respectively (Table 2) and hence affect the mitochondrial DNA (mtDNA) integrity, replication and transcription [28]. In line with this assumption it was documented that, ZnO nanoparticles induce mitochondrial dysfunction, morphological modification, and apoptosis [29].
In the present study, the enhanced expression of UCP2 in testicular tissues of rats treated with nanoparticles may facilitate the proton leak that may act as a possible control mechanism for mitochondrial ROS production in the testis, with a mild uncoupling leading to a reduction in mitochondrial membrane potential, which in turn reduces ROS production. However this defense mechanism may have a serious metabolic consequence as the protein leak decrease the metabolic efficiency of the mitochondria through uncoupling the oxidation from phosphorylation leading to decreased ATP production. The later effect may participate directly and/or indirectly in the apoptotic and necrotic cell death. In line with the present study, it was documented that, ZnO nanoparticle had a strong deleterious effect of mitochondrial function [30]. The mechanism by which nanoparticles induce the gene expression of UCP2 is unclear however we can postulate that it may a direct consequence of oxidative stress and increased free radicals and lipid peroxide which found in the present data (Tables 6-9).
Testicular genomic DNA showed very low or undetectable DNA laddering (DNA fragmentation) in the testes of the control rats (Fig. 1). Damage to DNA is a fundamental example of cellular toxicity, and it is critical to assess such damage for any nanoparticle that is likely to come in contact with humans, given that damage to DNA is highly correlated with an increased risk of cancer. Oguma et al [31] reported that the major function of p53 tumor suppressor is to curtail proliferation of abnormal cells. Their data suggest that low amount of ZnONPs (50 mM) is enough to trigger activation of p53 which in turn drives the expression of important downstream antioxidant genes. They observed upregulation of p53 and its associated genes after low concentration of H2O2 treatment. The present study showed enhancement in tumor suppressor p53 (p53), tumor necrosis factor-α (TNF-α) and interliukin-6 (IL-6) of male rats treated with aluminum oxide nanoparticles, zinc oxide nanoparticles and their combination. Park et al. [32] demonstrated that Al2O3NPs for caused significant increase in inflammatory cytokines IL-6. Also, Rodrigo et al. [33] reported that aluminum nanoparticles have been shown to initiate inflammatory events in macrophages, including secretion of pro-inflammatory cytokines.
The present study showed that exposure to nanoparticles caused deteriorations in semen characteristics and imbalance in the reproductive hormones (Testosterone, FSH, LH) and thyroid hormones (TSH, T3 and T4) and this may be due to the increase the levels of free radicals and nitric oxide in testes and the reduction in the antioxidants (Tables 6-9). Dadong et al. [34] demonstrated that ZnO nanoparticles could decrease mitochondrial membrane potential, increase the production of oxygen free radicals (ROS) and lead to the over-expression of genes that activate caspase 12 in RGC-5 cells. Because the increase of oxygen free radicals lead to the production of oxidative stress in endoplasmic reticulum membrane and induce apoptosis and necrosis by expression of caspase 12 and finally cause cell death. ROS and membrane lipoperoxidation of Sertoli cells and the damage in Sertoli and leydig cells lead to imbalance in FSH, LH and test hormones. Naher et al. [35] showed an association with low antioxidant level in the semen and bad quality of spermatozoa of the infertile subjects. Also, decreased antioxidant level in the seminal fluid has a negative impact on semen quality. In addition, Aitken et al. [36] reported that infertile males who that produce high levels of ROS have a five-fold less chance of initiating a pregnancy than infertile males who produce low levels of ROS. The increase in TBARS can bring negative effects on motility, sperm–oocyte fusion, and induces midpiece abnormalities [37].
Shirvani et al. [38] reported that ZnO nanoparticles have impact on Sertoli cells, occurrence of vacuolation phenomenon, apoptosis and reduction in various cell lines including spermatogonia, round and elongated spermatids. According to Bockelheide et al. [39], the presence of vacuoles in the cytoplasm of Sertoli cells indicate direct harm of nanoparticles on these cells, such that one of the obvious tissue damage signs can be the presence of vacuoles in Sertoli cells cytoplasm and between cell lines of seminiferous tubules. Yoshida et al. [40] found degeneration in Leydig cell and damage in the seminiferous tubules due to exposure to nanoparticles. Increase in the activity of testicular 17β-HSD and decrease in the activity of 17-KSR in the present study may lead to decrease of testosterone which may explain the reduction of serum testosterone levels. One of the indicators of the chemical toxicity on reproductive system is the decreased level of testosterone [40].
CONCLUSION
In conclusion, the obtained results revealed adverse effects of Al2O3NPs and ZnONPs alone and or their combination on the testicular architecture and caused fertility problems through different pathways including; changing gene expression of the proteins involved in the mitochondrial biogenesis and function (mtTFA and UCP2), induction of oxidative stress, lipid peroxidation, nitric oxide production, apoptotic and necrotic cell death pathways in the testicular tissues. Also, induction of inflammatory pathway through enhancing production of Tumor necrosis factor α and interleukin-6, disturbing production of tumor suppressor p53, sex hormones imbalance, and histopathological changes. Also, the present study showed that the reproductive toxicity of the combination of Al2O3NPs plus ZnONPs was more pronounced than each one.
REFERENCES
- Sadiq I M, Chowdhury B, Chandrasekaran N, Mukherjee A (2009) Antimicrobial sensitivity of Escherichia coli to alumina nanoparticles. Nanomedicine 5(3): 282-286.
- Khang D, Liu-Snyder P, Pareta R, Lu J, Webster TJ (2009) Reduced responses of macrophages on nanometer surface features of altered alumina crystalline phases. Acta Biomater 5(5): 1425-1432.
- Oesterling E, Chopra N, Gavalas V, Arzuaga X, Lim EJ, Sultana R, et al. (2008) Alumina nanoparticles induce expression of endothelial cell adhesion molecules. Toxicol Lett 178(3): 160-166.
- Prabhakar PV, Reddy UA, Singh SP, Balasubramanyam A, Rahman MF, Indu Kumari S, et al. (2012) Oxidative stress induced by aluminum oxide nanomaterial after acute oral treatment in Wistar rats. J Appl Toxicol 32(6): 436-445.
- Banasik A, Lankoff A, Piskulak A, Adamowska K, Lisowska H, Wojcik A (2005) Aluminum-induced micronuclei and apoptosis in human peripheral-blood lymphocytes treated during different phases of the cell cycle. Environmental toxicology 20(4): 402-406.
- Lima PD, Leite DS, Vasconcellos MC, Cavalcanti BC, Santos RA, Costa-Lotufo LV, et al. (2007) Genotoxic effects of aluminum chloride in cultured human lymphocytes treated in different phases of cell cycle. Food Chem Toxicol 45(7): 1154-1159.
- De Angelis I, Barone F, Zijno A, Bizzarri L, Russo MT, Pozzi R, et al. (2013) Comparative study of ZnO and TiO2 nanoparticles: physicochemical characterization and toxicological effects on human colon carcinoma cells. Nanotoxicology 7(8): 1361-1372.
- Saptarshi SR, Duschl A, Lopata AL. (2015) Biological reactivity of zinc oxide nanoparticles with mammalian test systems: an overview. Nanomedicine 10(13): 2075-2092.
- Sharma V, Singh S K, Anderson D, Tobin D J, Dhawan A. (2011) Zinc oxide nanoparticle induced genotoxicity in primary human epidermal keratinocytes. J Nanosci Nanotechnol 11(5): 3782-3788.
- Jingxia Z, Lanju X, Tao Z, Guogangc R, Zhuo Y (2009) Influences of nanoparticle zinc oxide on acutely isolated rat hippocampal CA3 pyramidal neurons. Neurotoxicol 30(2): 220-230.
- Park EJ, Kim H, Kim Y, Choi K (2011) Repeated-dose toxicity attributed to aluminum nanoparticles following 28-day oral administration, particularly on gene expression in mouse brain. Toxicological and Environ Chemistry 93(1): 120-133.
- Saman S, Moradhaseli S, Shokouhian A, Ghorbani M (2013) Histopathological effects of ZnO nanoparticles on liver and heart tissues in wistar rats. AdvBiores 4(2): 83-88.
- Piantadosi CA, Suliman HB (2006) Mitochondrial transcription factor A induction by redox activation of nuclear respiratory factor 1. J Biol Chem 281(1): 324-333.
- Berraondo B, Marti A, Duncan JS, Trayhurn P, Martinez JA (2000) Up-regulation of muscle UCP2 gene expression by a new b-adrenoceptor agonist, trecadrine, in obese (cafeteria) rodents, but down regulation in lean animals. Int J Obes Relat Metab Disord 24(2): 156-163.
- Miller SA, Dykes DD, Polesky HF (1988) A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic acids Res 16(3): 1215.
- Draper H, Hadley M (1990) Malondialdehyde determination as index of lipid peroxidation, Methods Enzymol 186: 421-431.
- Griffith OW (1980) Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal Biochem 106 (1): 207-212.
- Katryna B, Anita PP (1980) Purification of rat testicular microsomal 17-ketosteroid reductase. Evidence that 17-ketosteroid reductase and 17-bhydroxysteroid dehydrogenase are distinct enzymes. J Biol Chem 255(12): 5552-5559.
- Linder RE, Klinefetter GR, Strader LF, Narotsky MG, Suarez JD, Roberts NL, et al. (1995) Dibromoacetic acid affects reproductive competence and sperm quality in the male rats. Fundam Appl Toxicol 28(1): 9-17.
- Llobet JM, Colomina MT, Sirvent JJ, Domingo JL, Corbella J, Roberts NL, et al. (1995) Reproductive toxicology of aluminum in male mice. Fundam Appl Toxicol 25(1): 45-51.
- Blom E (1950) A one-minute live-dead sperm stain by means of eosinnigrosin. J Fertil Steril 1: 176-177.
- Drury RA, Wallington EA (1980) Carleton’s Histological Techniques. [6th Ed.] Oxford University Press, New York, Toronto, USA.
- Statistical Analysis System( SAS). (1998) SAS Procedure Guide. Release 6.03 Edition. SAS Institute Inc., Cary, NC, USA.
- Duncan DB (1955) Multiple ranges and multiple F tests. Biometrics 11(1): 1-42.
- Fernandez CDB, Porto EM, Arena AC, Kempinas WG (2008) Effects of altered epididymal sperm transit time on sperm quality. Int J Androl 31(4): 427-437.
- Sullivan R, Frenette G, Girouard J (2007) Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J Androl 9(4): 483-491.
- Malarkey CS, Bestwick M, Kuhlwilm JE, Shadel GS, Churchill ME (2011) Transcriptional activation by mitochondrial transcription factor A involves preferential distortion of promoter DNA. Nucleic Acids Res 40(2): 614-624.
- Chandrika C, Scott M, Bayne AV, Sykora P, Tian J, de Souza-Pinto NC, et al. (2010) The mitochondrial transcription factors A functions in mitochondrial base excision repair. DNA Repair 9(10): 1080-1089.
- Zhang XQ, Yin L H, Tang M, Pu Y P (2011) ZnO, TiO2, SiO2, and Al2O3 nanoparticles-induced toxic effects on human fetal lung fibroblasts. Biomed Environ Sci 24(6):661- 669.
- Filippi C, Pryde A, Cowan P, Lee T, Hayes P, Donaldson K, et al. (2015) Toxicology of ZnO and TiO2 nanoparticles on hepatocytes: Impact on metabolism and bioenergetics. Nanotoxicology 9(1): 126-134.
- Oguma K, Oshima H, Oshima M (2010) Inflammation, tumor necrosis factor and Wnt promotion in gastric cancer development. Future Oncol 6(4): 515-526.
- Park E, Sim J, Kim Y, Han BS, Yoon C, Lee S, et al. (2015) A 13-week repeated-dose oral toxicity and bioaccumulation of aluminum oxide nanoparticles in mice. Arch. Toxicol 89(3): 371-379.
- Rodrigo A, Valles G, Saldan L, Rodrı´guez M, Martı´nez M E, Munuera L, et al. (2006) Alumina particles influence the interactions of cocultured osteoblasts and macrophages. J Orthop Res 24(1): 46-54.
- Dadong G, Hongsheng BI, Qiuxin WU, Daoguang W, Yan C (2013) Zinc Oxide Nanoparticles Induce Rat Retinal Ganglion Cell Damage Through Bcl-2, Caspase-9 and Caspase-12 Pathways. J Nanosci Nanotechnol 13(6): 3769-3777.
- Naher ZU, Biswas SK, Mollah FH, Ali M, Arslan MI (2011) Role of Glutathione in Male Infertility. Bangladesh J Med Biochem 4(2): 20-25.
- Aitken RJ, Irvine DS, Wu FC (1991) Prospective analysis of sperm-oocyte fusion and reactive oxygen species generation as criteria for the diagnosis of infertility. Am J Obstet Gynecol 164(2): 542-551.
- Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN (2014) Oxidative stress and male reproductive health. Asian J Androl 16(1): 31-38.
- Shirvani H, Noori A, Mashayekh AM (2014) The Effect of ZnO Nanoparticles on the Growth and Puberty of Newborn Male Wistar Rats. Int J Basic Sci Appl Res 3: 180-185.
- Bockelheide K, Fleming SL, Johnson KJ, Patel SR, Schoenfeld HA (2000) Role of sertoli cells in injury associated testicular germ cell apoptosis. Exp Biol Med 225(2): 105-115.
- Yoshida M, Yoshida S, Sugawara L, Takeda K (2002) Matemal exposure to diesel exhaust decreases expression of steroidognic factor -1 and mulevian inhibiting of substance in the murine fetus. J Heal Sci 48: 317-324.
Copyright: Yousef MI, et al. © 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Yousef MI (2019). Reproductive Toxicity of Aluminum Oxide Nanoparticles and Zinc Oxide Nanoparticles in Male Rats. Nanoparticle 1(1): 3.
 Abstract
Abstract  PDF
PDF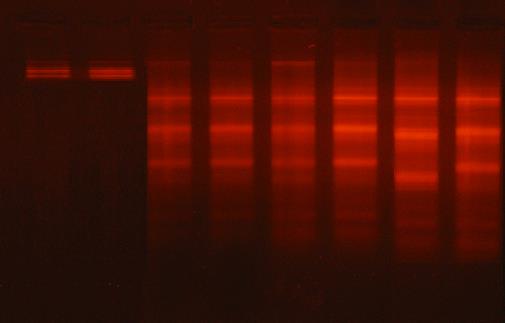
.jpg)
.jpg)
.jpg)