Past Issues
Protective Effects of Resveratrol Encapsulated In Liposomes or PLA Nanoparticles against Oxidative Stress in NRK-52E Cells
Kauther I Layas*, Prabal K. Chatterjee#, Ananth S Pannala#
*Libyan Company for Investing and Operating Health Utilities, Libyan Arab Jamahiriya
#Centre for Precision Health and Translational Medicine, School of Applied Sciences, University of Brighton, Brighton – BN2 4GJ, UK
*Corresponding author: Dr. Kauther I Layas: Libyan Company for Investing and Operating Health Utilities, Libyan Arab Jamahiriya. Tel: +218 217190100 E-mail: [email protected]
#Co author: Dr. Ananth S Pannala, Dr. Prabal K. Chatterjee: Centre for Precision Health and Translational Medicine, School of Applied Sciences, University of Brighton, Brighton – BN2 4GJ, UK
Citation: Layas K, et al. (2022). Protective Effects of Resveratrol Encapsulated In Liposomes or PLA Nanoparticles against Oxidative Stress in NRK-52E Cells. Nanoparticle. 3(1): 9.
Received: October 20, 2022 Published: November 9, 2022
Copyright: Layas K, et al. © (2022).
ABSTRACT
Liposomes and Polymeric nanoparticles are nanocarriers that can be used to enhance the pharmacokinetics of different drugs. The objective of the current study was to encapsulate resveratrol in DPPC liposomes and PLA nanoparticles; separately for the amelioration of PQ-induced oxidative stress on NRK-52E cells. Liposomes were produced by the hydration of a lipid film. Polymeric nanoparticles were prepared by double emulsification solvent diffusion method using PLA. They were then characterized for their particle size, zeta potential, drug loading, antioxidant activity, toxicity on NRK-52E cells, and protection against paraquat oxidation. The mean particle size for liposomes and PLA nanoparticles were respectively 197.4 ± 102.4 and 457.9 ± 56.1 nm and their surface zeta-potentials were -7.86 ± 6.36 and -21.6 ± 10.4 mV; respectively. LM and SEM images showed both types of nanoparticles to be spherical in shape. %LE efficiency for liposomes (12.04 ± 1.23%) was quite low compared to PLA nanoparticles (40.81 ± 29.14%). However, the % internalization for liposomes (5.95 ± 0.09%) was around 11 times higher than free resveratrol compared to PLA nanoparticles (0.93 ± 0.03%) which were only around two-fold higher. The toxicity tests showed that both liposomes and PLA nanoparticle forms of resveratrol showed less harmful effect on the cells than its free form mainly in the LDH assay. Furthermore, the benefit of encapsulation was also clear in the activity tests against PQ on the NRK-52E cell. From these results, it can be concluded that resveratrol-loaded liposomes and PLA nanoparticles can show more benefits over free resveratrol.
INTRODUCTION
Liposomes are nano-vesicles consisting of a liquid core surrounded by one or more bilayers of phospholipids [1]. It is becoming more and more possible to manipulate the composition and characteristics of liposomes to suit targeted applications [2]. Due to their phospholipid composition, they have the advantage of being pharmacologically inactive and are relatively safe [3]. Polymeric nanoparticles, on the other hand, are nano-sized solid colloidal particles consisting mainly of macromolecules. They can increase the solubility and stability of the carried drugs or reduce their side effects through targeted delivery [4].
The main drawback to the use of antioxidants such as resveratrol to treat oxidant-induced injury is usually their pharmacodynamic and pharmacokinetic properties, such as low solubility, low bioavailability, fast excretion, and to some extent, their toxicity at high doses [5]. The use of nanoparticles to deliver antioxidants is an extensively researched area. Several antioxidants such as vitamin E [6], resveratrol [7] [8] [9], and epicatechin [10] [11] have been developed as potential nanoparticle formulations to treat different oxidativestress related conditions such as pulmonary damage [6], pathophysiological aging [7] and cancer [10] [11] with varying degrees of success.
Resveratrol is a stilbene that despite its high antioxidant activity, a prospective study of about 800 elderlies, did not find any influence of dietary resveratrol on different agerelated diseases [12].
The purpose of this study was to enhance the bioavailability of resveratrol by encapsulating it into either liposomes or polymeric nanoparticles. The aim was to produce a preparation to reduce its toxicity and increase delivery into cells to ensure activity. The method used for the preparation of liposomes was based on the hydration of lipid film to prepare large multilamellar vesicles, followed by membrane extrusion to form smaller unilamellar vesicles. Polymeric nanoparticles were prepared by double emulsification solvent diffusion (DESD) method using polylactic acid (PLA). PLA nanoparticles and liposomes were characterized for particle size, zeta potential, morphology, loading efficiency (%LE) and drug delivery. Liposomes were also tested for their in vitro antioxidant activity using inhibition of tyrosine nitration by peroxynitrite and ABTS assay. The liposomes and PLA nanoparticles produced were also assessed for cellular toxicity and effectiveness for antioxidant activity against paraquat (PQ) oxidation using the NRK-52E cell line. The term nanoparticles in this research will refer to both liposomes and PLA nanoparticles, unless otherwise indicated.
MATERIALS AND METHODS
Materials
Trans-Resveratrol (>98%), 1,2-Dipalmitoyl-sn-glycero-3- phosphocholine (DPPC), PLA and other chemicals and solvent were purchased from Sigma-Aldrich Company Ltd (Poole, Dorset, UK. The NRK-52E cell line was purchased ECACC (Porton Down, Wiltshire, UK). All cell culture supplements and consumables such as Dulbecco’s Modified Eagle’s Medium (DMEM) were purchased from Fisher Scientific Ltd.
Preparation of DPPC liposomes
Liposomes were prepared by the hydration of a lipid film to prepare large multilamellar vesicles, followed by membrane extrusion [13]. The method was modified as required. 5 mg Resveratrol was dissolved in 5 mL DPPC solution (8 mg/ mL in ethanol). This solution was then evaporated using a rotatory evaporator at 40oC, 100 rpm under 100 mbar (IKA Rotary evaporator RV 3 V-C) to make a uniform lipid film. Deionized water (18.2 MΩ-cm) at 45°C was added to this film while shaking vigorously and sonicating for 10 min in an ultrasonic bath. An Avanti mini-extruder with 200 nm poresize polycarbonate membrane and glass air-tight syringes were used for extrusion. The whole system was heated to 40oC and the liposomal mixture was heated up to 60oC. Each aliquot of 1ml solution was then extruded by passing through the membrane 21 times while reducing temperature to below 41oC. Blank liposomes were prepared in the same manner. All liposomes were prepared in triplicates.
Preparation of PLA nanoparticles encapsulated with resveratrol
The method involved in the preparation of PLA nanoparticles in this study was DESD [14]. 200 mg PLA was dissolved in 10 mL DCM and added to 10 mL of 2%(w/v) polyvinyl alcohol (PVA) solution. An emulsion was made by mixing using a high-speed homogenizer (IKA® T25 digital Ultra-Turrax®) at 15000 rpm for 15 min. 10 mL acetone was added and further homogenized for a further 15 min. This mixture was then transferred into an excessive amount of water (100 mL) with continuous homogenizing for 15 min. Any organic solvent was then removed by using a rotatory evaporator, rotating at 130 rpm, heating at 40oC, and vacuum pressure 70 mbar. The solution was then left overnight to ensure complete removal of organic solvents. This solution was centrifuged (using a Sorvall RC-6 Plus Ultracentrifuge) at 15000 rpm and 4oC for 15 min. The supernatant was separated and kept for further investigation. Pellets (containing nanoparticles) were frozen at -80oC for at least one hour, after which were dried by lyophilization (Benchtop, 8L, -50°C, PTFE-Coated Collector, Labconco). Samples were then kept in fridge for further characterization.
Determination of nanoparticles morphology
Particle size distribution and surface morphology of the liposomes were investigated by light microscope using a Motic™ BA210E Trinocular Compound Microscope. PLA nanoparticles were examined for their morphology using scanning electron microscopy (SEM). Samples were mounted aluminon pin stubs and coated with a platinum coat of 4-5 nm thickness using a sputtering coater (Quorum technologies Q150 ES sputter coater) and inserted into a ZEISS Sigma Field Emission Gun SEM with an Everhart Thornley-Secondary Electron detector for imaging (Zeiss Evo LS-15 SEM). The accelerating voltage used ranged between 5-10 kV with a working distance around 8.5 mm.
Determination of particle size and surface charge
The particle size and charge of the nanoparticles were determined by photon correlation spectroscopy (PCS) (Malvern Zetasizer Nanoseries, Nano-ZS690 Malvern Instruments, UK). Plastic disposable cuvette used for measuring particle size and folded capillary cuvettes, with gold plated electrodes for measuring zetapotential. Analysis was carried out in triplicates at 25°C.
Determination of %LE in the liposomes and PLA nanoparticles
The antioxidant was extracted from the nanoparticles into a suitable solvent and then analyzed by HPLC to determine the %LE of the antioxidant in the encapsulated liposomes and PLA nanoparticles. Agilent 1220 series, equipped with ClarityChromatography SW, DataApex software, Agilent 1220 pump system with degasser, manual injector with a 20 µL injection loop, and an Agilent 1220 UV-visible detector. A 5 μm Fortis c18, 150 * 4.6 mm column was used with ethanol: water (50:50) as mobile phase, at a flow rate of 1 ml/min at 25°C with a detector wavelength of 300 nm. Standard concentrations of all the drugs over the range of 0.1–20 µg/ml were prepared. %LE was calculated as described by Li et al. [15].
In-vitro determination of antioxidant activity
ABTS decolorization assay
The assay was carried out by the interaction of different pure antioxidants and antioxidants encapsulated in liposomes with the ABTS radical cation, prepared as described by Re et al. [16]. For testing the free resveratrol, the radical solution was diluted in ethanol and for testing the liposomes it was diluted in deionised water to obtain an absorbance of 0.7 ± 0.02 at 734 nm.
Absorbance was measured after exactly 6 min of initial mixing, using a PerkinElmer UV-visible spectrophotometer with single cell holder. All determinations were carried out in triplicates. Trolox solutions with a concentration between 0-15µl was used as a standard [16]. Appropriate solvent blanks were tested in each assay. The %inhibition in the absorbance was calculated and plotted against concentration of resveratrol and the TEAC value was calculated.
Inhibition of tyrosine nitration by peroxynitrite
Peroynitrite preparation and the tyrosine nitration test were carried out as described by Pannala et al. [17]. For the current assay, only freshly prepared peroxynitrite was used. 50 µl of peroxynitrite (500 µM final concentration) was added to a solution of tyrosine (100 µM final concentration) in the presence of different concentration of liposomes encapsulated with antioxidants in 0.2 M phosphate buffer, pH7 to give a final volume of 1 ml. Appropriate blanks were carried out. Samples were then analysed by HPLC using Fortis C18 column (25 cm x 4.6 mm) on an Agilent 1260 system with a Quaternary pump, an autoinjector, an auto sampler and a diode array detector monitored at 350 nm. The system was equipped with Chem Station software. The mobile phase was 50 mM phosphate buffer, pH7: acetonitrile at a ratio of 95:5 v/v, at 1ml/min flow rate. The %inhibition in 3-NT was then plotted against theoretical concentration of antioxidant in liposomes. Different concentrations of peroxynitrite solutions (0.05-1 mM) diluted in 0.1 M NaOH were added to tyrosine solution (100 µM final concentration) in 0.2 mM phosphate buffer and the concentration of 3-NT formed was determined using the same HPLC method.
Characterization of nanoparticles using rat tubular epithelium (NRK-52E) cells
NRK-52E cell culture
NRK-52E cells (passage number 15 – 27) were routinely cultured in a cell culture medium prepared from DMEM supplemented with 5% (v/v) FBS, PenStrep (100 units/ml) solution, and NEAA (CCM I). Cells were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 (which was used throughout this research).
Effect of resveratrol-loaded nanoparticles on cell viability
The cytotoxicity of free resveratrol and resveratrol encapsulated in nanoparticles was determined by conducting MTT and LDH assays [18] [19]. Cells were seeded at a cell density of 4000 cells/well in 24 well plates and cultured in CCM I for 24 h. Nanoparticle solutions diluted in CCM I, without FBS (CCM II) were added separately, to the wells at a concentration range of 0.5 and 5%v/v (500 µL/well). Solutions of resveratrol (0-10 mM) were prepared in cell culture medium II, using ethanol initially, to dissolve the hydrophobic drug. The final measurements carried out in duplicates using Multiskan Ascent, v123. Tests were repeated in replicates of six.
Effect of resveratrol-loaded liposomes and PLA nanoparticles on paraquat (PQ) toxicity
PQ was used to induce injury to NRK-52E cells in vitro. The CCM I was removed and 500 µL of different concentrations of PQ (0-1 mM and 0-10 mM) prepared in CCM II was added. The cells were then incubated for 24 hours. The cells were then inspected under light microscope and tested using MTT and LDH assay. Tests were repeated in replicates of six.
To compare the effectiveness of free resveratrol and resveratrol loaded nanoparticles, they were tested for their ability to protect cells from PQ-induced oxidative stress. All three preparations were prepared and diluted in CCM II to make 6.25 & 62.5 µg/mL theoretical drug concentration, regardless of their %LE and % internalisation. This was to ensure all other variables such as PLA, PVA, and lipid concentrations were kept constant. The calculations, however, were made according to actual concentration of drug inside each preparation (table 1). NRK-52E cells were grown on 24-well plates, to 80- 90% confluence. The CCM I was removed and 500 µL of the two different concentrations of each antioxidant preparation prepared were added to the cells, separately. After 24 hours of incubation, the medium was removed and 500 µL of PQ was added at increasing concentrations (0, 0.2, 0.4, 0.6, 0.8, 1 mM). Cells were then tested after a further 24-hour incubation using the MTT and LDH assays. All tests were repeated in replicates of six.
RESULTS
Preparation and characterization of liposomes and PLA nanoparticles for their morphology
Both blank and resveratrol-loaded liposomes were successfully prepared in liquid form. Each passage of the liposomal solution through the extruder produced a clearer solution. They were then inspected under light microscope for their outer morphology, showing small spherical shapes (less than 400 nm). Moreover, the PLA nanoparticles were prepared and stored in dry form after lyophilization producing a white fluffy powder. Morphological assessment of blank and resveratrolloaded PLA nanoparticles was carried out using SEM. Images showed round spherical shaped particles with wide size distribution. There was no evidence of free drug crystals shown [Figure 1].
Figure 1: SEM images of PLA nanoparticles: Blank (left) and resveratrol-loaded PLA nanoparticles (right).
Particle size analysis and surface charge
The mean particle size analysis of resveratrol-loaded liposomes and PLA nanoparticles revealed a size range of 197.4 ± 102.4 & 457.9 ± 56.1 nm; respectively and a polydispersity index (PdI) of 0.222 & 1; respectively. The blank liposomes and PLA nanoparticles however, showed a size range of 194.9 ± 114.0 and 345.0 ± 98.7 nm; respectively and a PdI of 0.256 & 1; respectively.
Resveratrol-loaded liposomes and PLA nanoparticles showed a zeta potential of -7.9 ± 6.36 & -21.6 ± 10.40 mV; respectively. This was slightly less negative than their respective blank formulation which showed a zetapotential of 10.9 ± 3.45 & -38.0 ± 3.85 mV; respectively.
Determination of drug loading efficiency and cell internalisation
The % LE for liposomes (12.04 ± 1.23%) was quite low compared to PLA nanoparticles (40.81 ± 29.14%). However, the % internalisation for liposomes (5.95 ± 0.09%) was around 11 times higher than free resveratrol compared to PLA nanoparticles (0.93 ± 0.03%) which were only around two-fold higher.
In-vitro determination of antioxidant activity
ABTS decolorization assay
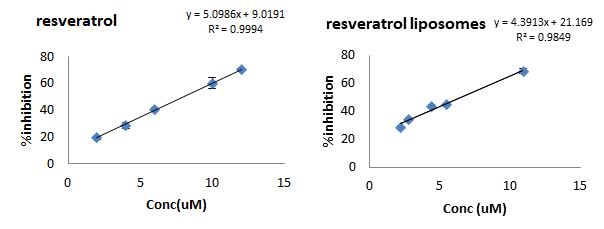
The extent in the decolorization of the ABTS+ by a resveratrol solution and the prepared resveratrol-loaded liposomes was calculated as the %inhibition in the absorbance and plotted as a function of the concentration of each resveratrol used [Figure 2]. Due to the solid nature of the PLA nanoparticles, this test was not applied. The TEAC value for free resveratrol and resveratrol-loaded liposomal preparation was 1.35 ± 0.04 & 1.18 ± 0.06; respectively. Blank liposomes were found to have negligible activity with a TEAC value of 0.034.
Figure 2: ABTS activity test. The %decolourisation to the ABTS solution by adding increased concentration of resveratrol solution (left) and resveratrol-loaded liposomes (right)
Tyrosine nitration assay
The addition of peroxynitrite to the tyrosine solution, resulted in the formation of 3-NT in an almost linear relationship [Figure 3]. This was confirmed by analysing the chromatographs produced by HPLC.
Figure 3: Tyrosine nitration assay. Relationship between peroxynitrite concentration and 3-NT formation (mean ± SD, n=3).
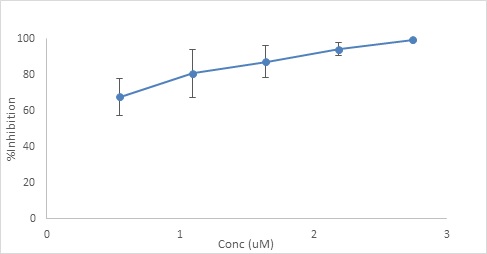
The ability of different concentrations of resveratrol-loaded liposomal solutions to inhibit the formation of 3-NT was determined [figure 4]. Results showed that all the resveratrolliposomes showed peoroxynitrite scavenging properties with 100% inhibition at high concentrations. A neglectable response was observed with blank liposomes, which did not exceed 13%, even at high concentrations.
Figure 4: The effects of different concentrations of different resveratrol-loaded liposomes on the concentration of 3-NT formed in the tyrosine nitration assay (mean ± SD, n=3).
Effect of resveratrol-loaded liposomes and PLA nanoparticles on the viability of NRK-52E cells
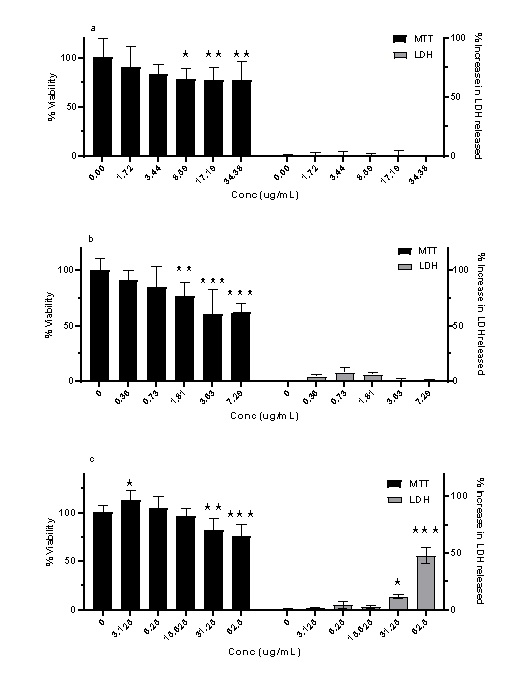
A range of equivalent concentrations of resveratrol solution and resveratrol-loaded liposomes and PLA nanoparticles were tested for their toxicity on NRK-52E cells [Figure 5]. The %viability decreased from 90.50% to 77.58% using resveratrolloaded liposomes (1.72 – 34.38 µg/mL) in a dose-dependent manner [Figure 5.a]. The %viability also decreased from 91.22% to 62.47% using resveratrol-loaded PLA nanoparticles [Figure 5.b] although, the resveratrol concentration was much lower (0.36 – 7.29 µg/mL). No significant increase in LDH release was observed using liposomes (0 – 1.38%) or PLA nanoparticles (0 – 7.82%). On the other hand, there was a noticeable rise in the %increase in LDH release (14.05 ± 2.26% & 56.36 ± 8.34%) using high concentrations of resveratrol solution (31.25 & 62.5 µg/mL; respectively). This was consistent with the reduction in %viability measured from the MTT assay (81.70 ± 12.47% & 75.48 ± 12.58%; respectively) using the same concentrations. Interestingly, using low concentrations of resveratrol solution (3.13 & 6.25 µg/mL) led to small increase in %viability (112.94 ± 10.25% & 104.79 ± 12.62%; respectively) [Figure 5.c].
Figure 5: The effect of increased concentration of resveratrol in three different forms on NRK-52E cells measured using two different assays: MTT and LDH. a. resveratrol-loaded liposomes. b. resveratrol-loaded PLA nanoparticles. c. resveratrol solution. (mean ± SD, N = 6, * p < 0.05, ** p < 0.01, *** p < 0.001 vs blank [0 µg/mL]). Data analysed using ordinary two-way ANOVA, followed by Bonferroni's multiple comparisons test.
Effect of resveratrol loaded liposomes and PLA nanoparticles on PQ toxicity
The relationship between the concentration of PQ was plotted against %LDH released and cell viability, obtained from LDH and MTT assays, respectively [Figure 6]. There was a significant increase in the %LDH with increased PQ concentration from 0.4 to 0.8 mM stabilised between 0.8 & 1 mM. These results correlate to the observations taken from microscopic examination, indicating that cell membrane starts to lose its integrity at 0.4 mM and cells undergo complete necrosis at 0.8 mM. MTT results showed an inverse response with percentage viability decreasing with increased concentration of PQ between 0.2 & 0.8 mM. There was also a slight increase in cell viability using 0.2 mM PQ compared to untreated cells.
Figure 6: PQ dose response graph. The effect of increased concentrations of PQ on %viability of, and %LDH released by, NRK-52E cells, mean ± SD, N = 6, p < 0.001 (using ordinary one-way ANOVA), **** p < 0.0001 compared to untreated cells (0 mM PQ) (using Bonferroni's post hoc test).
Resveratrol was prepared as a solution and as an encapsulated forms in liposomes and PLA nanoparticles. Details of the calculated concentrations within each sample, when considering the %LE are shown in table 1.
Table 1: The concentration of resveratrol in each sample and its given code (%LE Loading efficiency, PLA polylactic acid)
|
Drug |
Form |
Theoretical conc (µg/mL) |
%LE |
Actual conc (µg/mL) |
code |
|
Resveratrol |
Liposomes |
6.25 |
12.04% |
0.75 |
RL-1 |
|
Resveratrol |
Liposomes |
62.5 |
12.04% |
7.53 |
RL-2 |
|
Resveratrol |
PLA nanoparticles |
6.25 |
40% |
2.55 |
RP-1 |
|
Resveratrol |
PLA nanoparticles |
62.5 |
40.81% |
25.51 |
RP-2 |
|
Resveratrol |
solution |
6.25 |
NA |
6.25 |
RS-1 |
|
Resveratrol |
solution |
62.5 |
NA |
62.5 |
RS-2 |
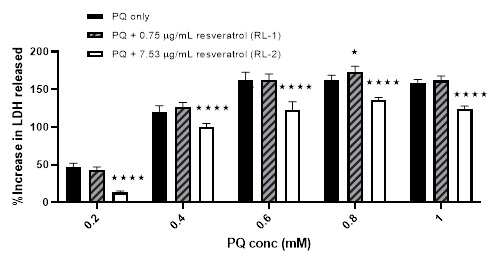
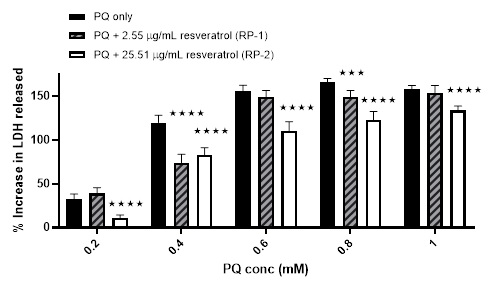
First, this harmful effect of PQ has been challenged using two concentrations of resveratrol-loaded liposomes. The %viability increased from 51.5 ± 10% using 0.4 mM PQ only to 76.74 ± 5.94% and 73.08 ± 11.49% after pre-incubating with RL-1 and RL-2; respectively. RL-1 continued to show protection even at higher concentration of PQ, although not as significant at 0.8 mM and 1 mM PQ. No significant effects were observed after pre-incubation with RL-2 using PQ concentrations ≥ 0.6 mM [Figure 7]. However, the results from the LDH assay showed more protection from the pre-incubation of RL2. The %increase in LDH release was lowered from 46.81 ± 5.38%, 120.57 ± 7.91%, 162.09 ± 10.59%, 161.84 ± 6.96%, and 159.09 ± 4.18% (using 0.2, 0.4, 0.6, 0.8 mM and 1 mM PQ only; respectively) to 13.23 ± 2.19%, 100.66 ± 4.23%, 122.37 ± 11.43, 136.12 ± 3.09% and 123.79 ± 4.33%; respectively after preincubating with RL-2 [Figure 8].
Figure 7: The effect of two different concentrations of resveratrol-loaded liposomes plus PQ compared to PQ only on %viability of cells using MTT assay. Mean ± SD, N = 6, p < 0.0001 (using twoway ANOVA), ** p < 0.01, **** p < 0.0001 compared to PQ only (using Bonferroni's post hoc test).
Figure 8: The effect of two different concentrations of resveratrol-loaded liposomes plus PQ compared to PQ only on the %increase in LDH release from cells. Mean ± SD, N = 6, p < 0.0001 (using two-way ANOVA), * p < 0.05, **** p < 0.0001 compared to PQ only (using Bonferroni's post hoc test).
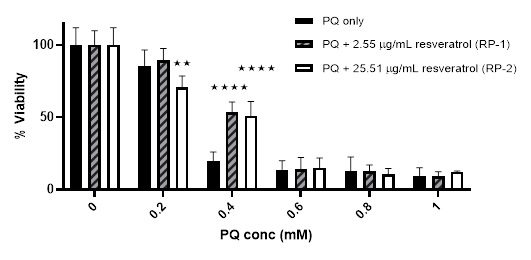
Secondly, the effect of PQ was also challenged using preincubation with resveratrol-loaded PLA nanoparticles. As with resveratrol-loaded liposomes, an increase in the %viability was noticed from 19.69 ± 6.19% using 0.4 mM PQ only to 53.29 ± 7.27% and 51.05 ± 9.97% when pre-incubating with RP-1 and RP-2; respectively. However, a slight reduction in %viability was noticed after pre-incubating with RP-2 and treating with 0.2 mM PQ (not noticed with RP-1). There was no effect on the %viability using either RP-1 or RP-2 after treating with higher concentrations of PQ (≥ 0.6 mM) [Figure 9]. In contrast to the effect of liposomes, pre-incubating with both strengths of resveratrol-loaded PLA nanoparticles showed protection in the LDH assay, although more evident with RP-2 [Figure 10].
Figure 9: The effect of two different concentrations of resveratrol-loaded PLA nanoparticles plus PQ compared to PQ only on %viability of cells using MTT assay. Mean ± SD, N = 6, p < 0.0001 (using two-way ANOVA), ** p < 0.01, **** p < 0.0001 compared to PQ only (using Bonferroni's post hoc test).
Figure 10: The effect of two different concentrations of resveratrol-loaded PLA nanoparticles plus PQ compared to PQ only on the %increase in LDH release from cells. Mean ± SD, N = 6, p < 0.0001 (using two-way ANOVA), *** p < 0.001, **** p < 0.0001 compared to PQ only (using Bonferroni's post hoc test).
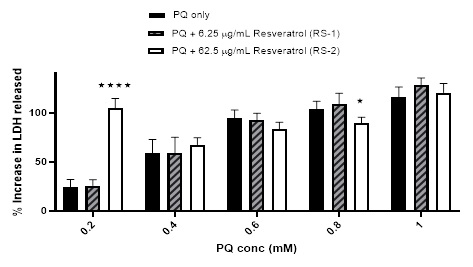
Finally, slightly different results were obtained when the cells were pre-incubated with resveratrol solution (un-encapsulated form) prior to treatment with PQ. Initially, the %viability was reduced from 104.05 ± 12.37% (using 0.2 mM PQ) to 91.49 ± 11.95 and 69.32 ± 6.4% when pre-incubating with RS-1 & RS2; respectively. This was followed by a rise in %viability from 28.74 ± 5.37% (using 0.6 mM PQ) to 63.43 ± 12.82% and 51.22 ± 11.76% when pre-incubating with RS-1 & RS-2; respectively. No obvious effect was noted from pre-incubation with these sample on the %viability caused by ≥ 0.6 mM PQ [Figure 11]. The harmful effect of RS-2 was also noticed in the LDH assay results using 0.2 mM PQ, although not noted with RS-1 with the %increase in LDH release leaping from 24.11 ± 7.75% using 0.2 mM PQ only to 104.83 ± 9.83% when pre-incubating with RS-2. A smaller increase from 59.13 ± 13.72% (using 0.4 mM PQ only) to 67.47 ± 7.02% was also observed when preincubating with same sample. Trivial reductions were also noted in the %increase in the LDH release caused by PQ when pre-incubating with RS-2. No major effect was observed using RS-1 in the LDH results [Figure 12].
Figure 11: The effect of two different concentrations of resveratrol solution plus PQ compared to PQ only on %viability of cells using MTT assay. Mean ± SD, N = 6, p < 0.0001 (using two-way ANOVA), ** p < 0.01, **** p < 0.0001 compared to PQ only (using Bonferroni's post hoc test).
Figure 12: The effect of two different concentrations of resveratrol solution plus PQ compared to PQ only on the %increase in LDH release from cells. Mean ± SD, N = 6, p < 0.0001 (using two-way ANOVA), * p < 0.05, **** p < 0.0001 compared to PQ only (using Bonferroni's post hoc test).
DISCUSSION
Resveratrol was encapsulated in both liposomes and PLA nanoparticles. DPPC was selected for its superior stability over other lipids. For example, paclitaxel-liposomes prepared from DPPC were more stable than when using DSPC or DMPC [20]. The extrusion step was performed to reduce the size of these liposomes. A membrane with a pore size of 200nm was selected. A comparative study to optimise the different parameters of extrusion, showed that membranes with pore size less than 200 nm forms liposomes slightly bigger than the nominal pore size [21]. Nevertheless, it has been recommended that filter pore size should be ≥ 200 nm, to extrude liposomes comfortably by a manual extruder. [22]. Previous studies showed that increasing the temperature of the initial solution does not affect size and polydispersity of final liposomes [21]. The same study demonstrated that increasing the number of cycles of passing through the extruder reduces the size and polydispersity of the product until a certain number of cycles where it levels off. For this reason, the solution was initially heated up and the number of passage cycles was maintained to 21 cycles for each batch.
The PLA nanoparticles were prepared by adding resveratrol to the co-solvent acetone. However, others have managed to prepare resveratrol loaded-PLA and PLA-PEG nanoparticles via single-emulsion solvent evaporation technique using dichloromethane and 1%PVA with higher encapsulation efficiency (%EE) [23]. However, it was not used in this study due to its toxicity. Others published methods include simple desolvation to form resveratrol-loaded albumin nanoparticles [24] and coacervation process to form resveratrol-loaded casein nanoparticles [25].The PLA nanoparticles were slightly larger in size than the liposomes. This was attribute to both the preparation technique and the separation step (Ostwald ripening may have increased the size overnight) [26].
As noted from the results, resveratrol uptake has been increased by the encapsulation into both liposomes and PLA nanoparticles. Others also showed that encapsulating resveratrol into chitosan and γ-poly (glutamic acid) nanocapsules using ionic gelation increased cellular uptake by Caco-2 cells from 3.5% to around 6%. This was explained by the increased stability and solubility of resveratrol [27]. During the toxicity tests in the current study, both liposomes and PLA nanoparticle forms of resveratrol showed less harmful effect on the cells than its free form mainly in the LDH assay. Furthermore, the benefit of encapsulation was also clear in the activity tests against PQ on the NRK-52E cell. Resveratrol has recently been reported for its hormetic dose–response effect. This means that it has a dual action: cytoprotective at low doses and cytotoxic at high doses although the exact biological active concentration is yet to be determined [28]. The advantages of encapsulating resveratrol into nanoparticles such as increased stability, enhanced antioxidant activity, improved internalisation and decreased toxicity has been discussed thoroughly with several examples and possible advantages by Chung et al., 2020. It is. Ideally, encapsulating resveratrol helps delivering the drug and allow slow release to achieve optimum activity without toxic levels being reached [29].
In the current study, there was some protection against PQ observed after pre-incubation with resveratrol solutions and was also shown in previous reports [30]. However, at a low concentration of PQ, the resveratrol solution showed enhanced harmful effects on the cells, which was more evident with higher concentrations of resveratrol. Nevertheless, the encapsulated forms of resveratrol did not show any detrimental effect on the cells. This was attributed to the reduction in the toxicity of resveratrol due to its encapsulation. Shi et al. have also reported the improved antioxidant activity of resveratrol in its nanoparticle form and showed that the encapsulation of resveratrol into zien/sodium hyaluronate nanoparticles improves its in vitro antioxidant and antitumor activities [31]. Furthermore, the encapsulation of resveratrol into β-lactoglobulin nanoparticles also showed improved in vitro antioxidant activity against H2O2 treatment in human type-2 alveolar epithelial cells compared to its native form [32].
CONCLUSIONS
In summary, resveratrol was successfully encapsulated into liposomes and PLA nanoparticles. The liposomes characterized in this study possessed a narrow size distribution while the PLA nanoparticles were slightly larger and a wider size distribution. An acceptable zeta potential with good drug-loading efficiency for both liposomes and PLA nanoparticles was achieved making them suitable to be developed into future formulations. Cytotoxicity studies showed the formulations to be well-tolerated by NRK-52E cells at clinically relevant concentrations. Additionally, the activity studies against the harmful effect of PQ showed the benefits of encapsulation over free drug form.
REFERENCES
1. Ria M and Kon K. (2015). Nanotechnology in Diagnosis, Treatment and Prophylaxis of Infectious Diseases. Academic Press. p. 133-149.
2. Banerjee R. (2001). Liposomes: Application in Medicine,” Journal of Biomaterials Applications. J Biomater Appl. 16(1): 3-21.
3. Sercombe L, Veerati T, Moheimani F, Wu S, Sood A, Hua S. (2015). Advances and Challenges of Liposome Assisted Drug Delivery. Front Pharmacol. 6: 286.
4. Selby L, Cortez-Jugo C, Such G, Johnston A. (2016). Nanoescapology: Progress towards understanding the endosomal escape of polymeric nanoparticles. WIREs. Nanomedicine and nanobiotechnology. 9(5): e1452.
5. Suntres Z. (2011). Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J Toxicol. 2011: 152474.
6. Galvao A, Galvao J, Pereira M, Cadena P, Santos M, Fink M, et al. (2016). Cationic liposomes containing antioxidants reduces pulmonary injury in experimental model of sepsis: Liposomes antioxidants reduces pulmonary damage. Respir Physiol Neurobiol. 231: 55-62.
7. Csiszar A, Pinto T, Gautam T, Kleusch C, Hoffmann B and Ungvari Z. Resveratrol encapsulated in novel fusogenic liposomes activates Nrf2 and attenuates oxidative stress in cerebromicrovascular endothelial cells from aged rats. J Gerontol A Biol Sci Med Sci. vol. 70(3): 303-313.
8. Bonechi C, Martini S, Ciani L, Lamponi S, Rebmann H, Rossi C, et al. (2012). Using liposomes as carriers for polyphenolic compounds: the case of trans-resveratrol. PLoS One. 7(8): e41438.
9. Isailovi B, Kostic I, Zvonar A, Dordevic V, Gasperlin M, Nedovic N, et al. (2013). Resveratrol loaded liposomes produced by different techniques. Innovative Food Science & Emerging Technologies. 19: 181-189.
10. Fang J, Hung C, Hwang T and Huang Y. (2005). Physicochemical characteristics and in-vivo deposition of liposome-encapsulated tea catechins by topical and intratumor administrations. J Drug Target. 13(1): 19-27.
11. Fang J, Lee W, Shen S and Huang Y. (2006). Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas J Dermatol Sci. 42(2): 101-109.
12. Semba R, Ferrucci L, Bartali B, Urpí-Sarda M, Zamora-Ros R, Sun K, et al. (2014). Resveratrol Levels and All-Cause Mortality in Older Community-Dwelling Adults. JAMA Intern Med. 174(7): 1077-1084.
13. Dichello G, Fukuda T, Maekawa T, Whitby R, Mikhalovsky S, Alavijeh M, et al. (2017). Preparation of liposomes containing small gold nanoparticles using electrostatic interactions. Eur J Pharm Sci. 105: 55-63.
14. Buhecha M, Lansley B, Somavarapu S, Pannala S. (2019). Development and characterization of PLA nanoparticles for pulmonary drug delivery: Co-encapsulation of theophylline and budesonide, a hydrophilic and lipophilic drug, Journal of Drug Delivery Science and Technology. 53: 101128.
15. Li Z, Liu K, Sun P, Mei L, Hao T, Tian Y, et al. (2013) Poly (D, L-lactide-co-glycolide)/montmorillonite nanoparticles for improved oral delivery of exemestane. J Microencapsul. 30(5): 432-440.
16. Re R, Pelleggente N, Proteggente A, Pannala A, Yang M and Ricde-Evans C. (1998). Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 26(9-10): 1231-1237.
17. Pannala A, Rice-Evans C, Halliwell B and Singh S. (1997). Inhibition of Peroxynitrite-Mediated Tyrosine. Biochemical and Biophysical Research Communications. 232(1): 164- 168.
18. Abe K and Matsuki N. (2000). Measurement of cellular 3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) reduction activity and lactate dehydrogenase release using MTT. Neurosci Res. 38(4): 325-329.
19. Mosmann T. (1983). Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65(1-2): 55-63.
20. Campbell R, Balasubramanian S and Straubinger R. (2000). Influence of cationic lipids on the stability and membrane properties of paclitaxel-containing liposomes. J Pharm Sci. 90(8): 1091-105.
21. Ong S, Chitneni M, Lee K, Ming L and Yuen K. (2016). Evaluation of extrusion technique for nanosizing liposomes. Pharmaceutics. 8(4): 36.
22. Hope M, Nayer L, Mayer L and Cullis P. (1993). Reduction of lipsome size and preparation of unilamellar vesicles by extrusion techniques, in Liposome technology. CRC Press. 123-126.
23. Lindner G, Khalil N and Mainardes R. (2013). Resveratrolloaded polymeric nanoparticles: Validation of an HPLCPDA method to determine the drug entrapment and evaluation of its antioxidant activity. Scientific World Journal. 506083.
24. Geng T, Zhao X, Ma M, Zhu G and Yin L. (2017). Resveratrolloaded albumin nanoparticles with prolonged blood circulation and improved biocompatibility for high effective targeted pancreatic tumour therapy. Nanoscale Res Lett. 12(1): 437.
25. Penalva R, Morales J, Gonzaez-Navarro J, Larraneta E, Quincoces G, Penuelas I, et al. (2018). Increased oral bioavailability of resveratrol by its encapsulation in casein nanoparticles. Int J Mol Sci. 19(9): 2816.
26. Liu B and Hu X. (2020). Hollow micro- and nanomaterials: Synthesis and applications in Micro and Nano Technologies. Advanced Nanomaterials for Pollutant Sensing and Environmental Catalysis. Elsevier. 1-38.
27. Jeon Y, Lee J and Lee H, (2016). Improving solubility, stability, and cellular uptake of resveratrol by nanoencapsulation with chitosan and gamma-poly (glutamic acid). Colloids Surf B Biointerfaces. 147: 224-233.
28. Shaito A, Posadino A, Younes N, Hasan H, Halabi S, Alhababi D, et al. (2020). Potential adverse effects of resveratrol: A litrature review. Int J Mol Sci. 21(6): 2084.
29. Chung I, Subramanian U, Thirupathi P, Venkidasamy B, Samynathan R, Gangadher B, et al. (2020). Resveratrol nanoparticles: A promising therapeuitic advancment over native resveratrol. Processes. 8(4): 458.
30. He X, Wang L, Szclarz G, Bi Y and Ma Q. (2012). Resveratrol inhibits paraquat-induced oxidative stress and fibrogenic response by activating the nuclear factor erythroid 2-related factor 2 pathway. J Pharmacol Exp Ther. 342(1): 81-90.
31. Shi Q, Wang X, Tang X, Zhen N, Wang Y, Luo A, et al. (2021). In vitro antioxidant and antitumor study of zein/SHA nanoparticles loaded with resveratrol. Food Sci Nutr. 9(7): 3530-3537.
32. Kim J, Park E, Ha H, Jo C, Lee W, Lee S, et al. (2016). Resveratrol loaded nanoparticles induce antioxidant activity against oxidative stress. Asian-Australas J Anim Sci, 29(2): 288-298.
33. Bulbake U, Doppalapudi S, Kommineni N and Khan W. (2017). Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics. 9(2): 12.
34. Mayer B. (2015). Encyclopedia of Nephrology and Acute Kidney Injury. Foster Academics. 147-163.
35. Wu J, Pan X, Fu H, Zheng Y, Dai Y, Yin Y, et al. (2017). Effect of curcumin on glycerol-induced acute kidney injury in rats. Sci Rep. 7(1): 10114.
36. Pavlakou P, Zhang H, O'Connor Z, Chertow M, Crowley T, Choudhury D, et al. (2008). Intensity of Renal Support in Critically Ill Patients with Acute Renal Injury. N Engl J Med. 359(1): 7-20.
37. Burns J, Yokota T, Ashihara H, Lean M and Crozier A. (2002). Plant foods and herbal sources of resveratrol. J Agric Food Chem. 50(11): 3337-3340.
38. Qin J, Chen D, Lu W, Xu H, Yan C, HU H, et al. (2008). Preparation, Characterization, and Evaluation of Liposomal Ferulic Acid In Vitro and In Vivo. Drug Dev Ind Pharm. 34(6): 602-608.
39. Crowell J, Korytko P, Morrissey R, Booth T and Levine B. (2004). Resveratrol-associated renal toxicity. Toxicol Sci. vol. 82(2): 614-619.
40. Li J, Wang X, Zhang T, Wang C, Huang Z, Luo X, et al. (2015). A review on phospholipids and their main applications in drug delivery systems. Asian Journal of Pharmaceutical Sciences. 10(2): 81-98.
 Abstract
Abstract  PDF
PDF.JPG)
.jpg)
.jpg)